
���Ȼ���(SCl2)�۵㣭78�棬�е�59�棬�ܶ�1.638 g/mL����ˮ�ֽ⣬���Ȼ����������������ÿ�������Ҫ�����Լ���������(SOCl2)����������������ϳɶ��Ȼ����ʵ��װ�ã�
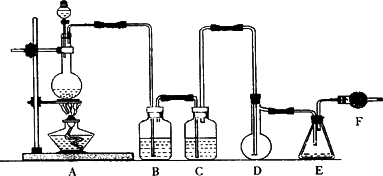
�Իش��������⣺
1��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ________��
2��װ��B��CӦʢ�ŵ�ҩƷ�ֱ���________��________��
3��ʵ�鿪ʼǰ����D�з�һ��������ۣ�����ʹ���ۻ���Ȼ��ת����ҡ����ƿʹ��������ƿ�ڱ��γ�һ������棬��������Ŀ����________��
4��ʵ��ʱ��Dװ���������50��59�森��ò��õĴ�ʩ��________����η�ֹE��Һ��ӷ���________��
5��Fװ���и��������ʢ������________��������________��
6���ɶ��Ȼ�����SO3���������������ȵĻ�ѧ����ʽΪ________��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�ش��������⣺
(1)B�з�����Ӧ�����ӷ���ʽΪ_____________________��
(2)C��ʢ�ŵ��Լ���______________��D���Լ���������________________________��
(3)ʵ�鿪ʼǰ������E�з���һ��������ۣ�������
(4)E�з�����Ӧ�Ļ�ѧ����ʽΪ________________________��
(5)���Ȼ���������������һ�������·�Ӧ�����������ȣ�������һ�ִ�����Ⱦ�д��������Ӧ�Ļ�ѧ����ʽ____________________________________��
(6)����ʵ��װ���У���һ�����Բ�����֮����Ҫ�Ľ��䣬������Ľ���Ĵ�ʩ________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣����Ȼ���(SCl2)�۵㡪78oC���е�59 oC���ܶ�1.638g/cm3����ˮ�ֽ⣬����������������Ӧ�ϳɶ��Ȼ����ʵ��װ�ã�����F��װ����ˮCaCl2���塣
�Իش��������⣺
��1��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ��
��2��װ��C��ʢ�ŵ��Լ��� ��װ��F������Ϊ
��3��ʵ�鿪ʼǰ���ž�ϵͳ�п�����������Ŀ����
����D�з���һ��������ۣ�����ʹ֮�ڻ���Ȼ��ҡ����ƿʹ��������ƿ�ڱ��γ�һ����Ĥ����������Ŀ����
��4��ʵ��ʱ����η�ֹE��Һ��ӷ�
��5���������߿�����F���ӵ���������ָ������ʢװ�Լ����ƣ������Ƹ�ʵ��װ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��2011ѧ�갲��ʡʦ���и�һ��ѧ�����п��黯ѧ�Ծ� ���ͣ�ʵ����
��12�֣����Ȼ���(SCl2)�۵㡪78oC���е�59 oC���ܶ�1.638g/cm3����ˮ�ֽ⣬����������������Ӧ�ϳɶ��Ȼ����ʵ��װ�ã�����F��װ����ˮCaCl2���塣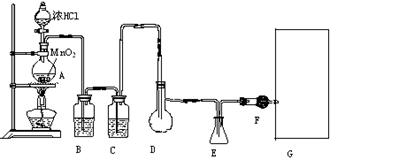
�Իش��������⣺
��1��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ�� 
��2��װ��C��ʢ�ŵ��Լ��� ��װ��F������Ϊ
��3��ʵ�鿪ʼǰ���ž�ϵͳ�п�����������Ŀ����
����D�з���һ��������ۣ�����ʹ֮�ڻ���Ȼ��ҡ����ƿʹ��������ƿ�ڱ��γ�һ����Ĥ����������Ŀ����
��4��ʵ��ʱ����η�ֹE��Һ��ӷ�
��5���������߿�����F���ӵ���������ָ������ʢװ�Լ����ƣ������Ƹ�ʵ��װ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���Ϻ��мζ���������ѧ����ĩ��ѧ�������л�ѧ�Ծ��������棩 ���ͣ�ʵ����
���Ȼ���(SCl2)�۵�-78�棬�е�59�棬�ܶ�1��638g��mL����ˮ�ֽ⣬���Ȼ����������������ÿ�������Ҫ�����Լ���������(SOCl2)����������������ϳɶ��Ȼ����ʵ��װ�á�
�Իش��������⣺
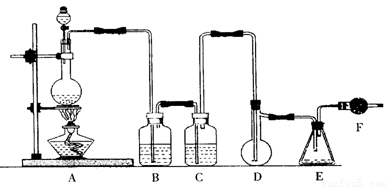
��1��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2��װ��B��CӦʢ�ŵ�ҩƷ�ֱ��� �� ��
��3��ʵ�鿪ʼǰ����D�з�һ��������ۣ�����ʹ���ۻ���Ȼ��ת����ҡ����ƿʹ��������ƿ�ڱ��γ�һ������棬��������Ŀ���� ��
��4��ʵ��ʱ��Dװ���������50��59�森��ò��õĴ�ʩ�� ����η�ֹE��Һ��ӷ��� ��
��5��Fװ���и��������ʢ������ �������� ��
��6���ɶ��Ȼ�����SO3���������������ȵĻ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�찲��ʡ��һ��ѧ�����п��黯ѧ�Ծ� ���ͣ�ʵ����
��12�֣����Ȼ���(SCl2)�۵㡪78oC���е�59 oC���ܶ�1.638g/cm3����ˮ�ֽ⣬����������������Ӧ�ϳɶ��Ȼ����ʵ��װ�ã�����F��װ����ˮCaCl2���塣
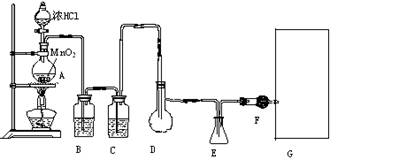
�Իش��������⣺
��1��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ��
��2��װ��C��ʢ�ŵ��Լ��� ��װ��F������Ϊ
��3��ʵ�鿪ʼǰ���ž�ϵͳ�п�����������Ŀ����
����D�з���һ��������ۣ�����ʹ֮�ڻ���Ȼ��ҡ����ƿʹ��������ƿ�ڱ��γ�һ����Ĥ����������Ŀ����
��4��ʵ��ʱ����η�ֹE��Һ��ӷ�
��5���������߿�����F���ӵ���������ָ������ʢװ�Լ����ƣ������Ƹ�ʵ��װ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com