
��14�֣�����̼���ƺ��Ȼ��ƵĹ������Ϊ�˲ⶨ��Ʒ��̼���Ƶĺ�������ѧ��ȤС��ͬѧ���������������ʵ�顣
��һ��
ʵ��װ��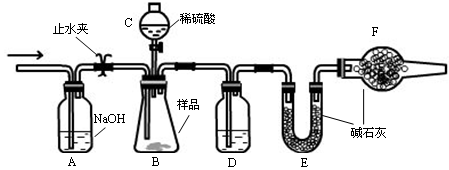
ʵ�鲽��
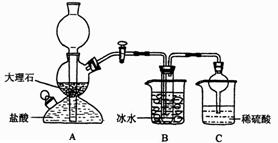
�����Ӻ�װ�ã���������ԣ�
��ʢװҩƷ��������Ʒa g������E�����������Ӻ�װ�ã�
�۹ر�ֹˮ�У���B�м���һ����ϡ���
�ܵ�B�г�ַ�Ӧ��ֹˮ�У�����Aװ�ã�ͨ��һ�����Ŀ�������B��D�в��������ȫ�����뵽Eװ���У�
�ݳ���E��������b g ��
�ش��������⣺
��1������C������ ��D��ʢ�ŵ��Լ��� ��
��2��B�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��3��A�з�Ӧ�����ӷ���ʽΪ ��
��4���ı�����һ�����������²ⶨ��Ʒ�е�̼���Ƶ������ٷֺ���ƫ�͵��� ��ѡ����ĸ��
A.ʵ����ڲ�ͨ����� B.������C�е����ỻ������
C.��������D D.��������F
�ڶ���
ʵ��װ��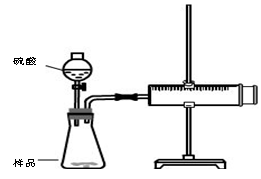
ʵ�鲽��
�ټ��װ��������
��װ���Լ���������Ʒb g,���Ӻ�װ�ã���ע�����Ļ����Ƶ��ף���0mL��
�ۼ���20mL���ᣬ
�ܴ���Ӧ��ֽ��к��������ƶ������»���ǰ�˶�Ӧ�Ŀ̶�ΪV mL
�ش���������
����װ�������Եķ����ǣ� ��
��2�����ڲ����������������ֹ۵㣬�۵�һ��������������ΪVmL���۵����������������Ϊ(V-20)mL������Ϊ ����۵�һ�����۵��������ȷ��
��3�������ʵ���ڱ�״���½��У�b=0.5g��V=76mL������Ʒ��̼���Ƶ������ٷֺ���Ϊ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ̼��������������������ԭ���κ��յȵ�ԭ���Ậ��Na2SO4��NaCl�е�һ�ֻ��������ʡ����й�ҵ̼������Ʒ��ij��ѧ��ȤС���ҵ̼�������Ƿ������������ʽ���̽����ʵ���������µ�������ҩƷ�ɹ�ʹ�ã�
�ձ����Թܡ���������ҩ�ס��ιܡ��ƾ��ơ��ԹܼУ�1.0 mol��L-1H2SO4��1.0 mol��L-1HNO3��1.0mol��L-1HCl��NaOHϡ��Һ��0.1 mol��L-1AgNO3��0.1 mol��L-1BaCl2 ��0.1 mol��LBa(NO3)2������ˮ�������Dz���ʵ��̽�����̣�
1��������裺
����1 ��ɫ��ĩΪ �Ļ���
����2 ��ɫ��ĩΪ �Ļ���
����3 ��ɫ��ĩΪ̼���ơ������ơ��Ȼ��ƵĻ���
2)���ڼ������ʵ�鷽��
3)����ʵ�鷽������ʵ��
��ش�
��1��ijͬѧȡ������Ʒ���Թ��У�����������ˮ�ܽ⣬���Թ��е���0.1 mol��L-1��BaCl2��Һ���а�ɫ��������������Ϊ��Ʒ�к���Na2SO4������Ϊ���Ľ�����������������Եġ����ġ�����ԭ���ǣ� ��
��2�����ʵ�鷽��
���ڹ�ҵ̼�������������ʾ�������һ���裬��Ƴ�ʵ�鷽�����ڴ�������𣩡�
| ��� | ʵ����� | Ԥ������ͽ��� |
| �� |
| ����ɫ���������˵����Ʒ�к���Na2CO3�� �������������˵����Ʒ��û��Na2CO3��
|
| �� |
|
|
| �� |
|
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣�����̼���ƺ��Ȼ��ƵĹ������Ϊ�˲ⶨ��Ʒ��̼���Ƶĺ�������ѧ��ȤС��ͬѧ���������������ʵ�顣
��һ��
ʵ��װ��
ʵ�鲽��
�����Ӻ�װ�ã���������ԣ�
��ʢװҩƷ��������Ʒa g������E�����������Ӻ�װ�ã�
�۹ر�ֹˮ�У���B�м���һ����ϡ���
�ܵ�B�г�ַ�Ӧ��ֹˮ�У�����Aװ�ã�ͨ��һ�����Ŀ�������B��D�в��������ȫ�����뵽Eװ���У�
�ݳ���E��������b g ��
�ش��������⣺
��1������C������ ��D��ʢ�ŵ��Լ��� ��
��2��B�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��3��A�з�Ӧ�����ӷ���ʽΪ ��
��4���ı�����һ�����������²ⶨ��Ʒ�е�̼���Ƶ������ٷֺ���ƫ�͵��� ��ѡ����ĸ��
A.ʵ����ڲ�ͨ����� B.������C�е����ỻ������
C.��������D D.��������F
�ڶ���
ʵ��װ��
ʵ�鲽��
�ټ��װ��������
��װ���Լ���������Ʒb g,���Ӻ�װ�ã���ע�����Ļ����Ƶ��ף���0mL��
�ۼ���20mL���ᣬ
�ܴ���Ӧ��ֽ��к��������ƶ������»���ǰ�˶�Ӧ�Ŀ̶�ΪV mL
�ش���������
��1�� ����װ�������Եķ����ǣ� ��
��2�����ڲ����������������ֹ۵㣬�۵�һ��������������ΪVmL���۵����������������Ϊ(V-20)mL������Ϊ ����۵�һ�����۵��������ȷ��
��3�������ʵ���ڱ�״���½��У�b=0.5g��V=76mL������Ʒ��̼���Ƶ������ٷֺ���Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�켪��ʡ��һ����������⻯ѧ�Ծ� ���ͣ�ʵ����
��14�֣�����̼���ƺ��Ȼ��ƵĹ������Ϊ�˲ⶨ��Ʒ��̼���Ƶĺ�������ѧ��ȤС��ͬѧ���������������ʵ�顣
��һ��
ʵ��װ��
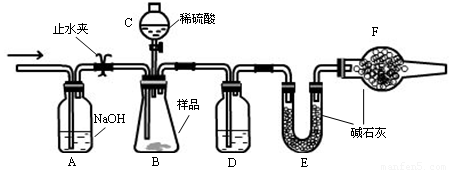
ʵ�鲽��
�����Ӻ�װ�ã���������ԣ�
��ʢװҩƷ��������Ʒa g������E�����������Ӻ�װ�ã�
�۹ر�ֹˮ�У���B�м���һ����ϡ���
�ܵ�B�г�ַ�Ӧ��ֹˮ�У�����Aװ�ã�ͨ��һ�����Ŀ�������B��D�в��������ȫ�����뵽Eװ���У�
�ݳ���E��������b g ��
�ش��������⣺
��1������C������ ��D��ʢ�ŵ��Լ��� ��
��2��B�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��3��A�з�Ӧ�����ӷ���ʽΪ ��
��4���ı�����һ�����������²ⶨ��Ʒ�е�̼���Ƶ������ٷֺ���ƫ�͵��� ��ѡ����ĸ��
A.ʵ����ڲ�ͨ����� B.������C�е����ỻ������
C.��������D D.��������F
�ڶ���
ʵ��װ��
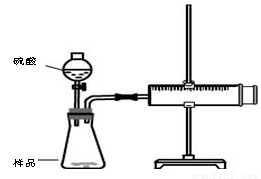
ʵ�鲽��
�ټ��װ��������
��װ���Լ���������Ʒb g,���Ӻ�װ�ã���ע�����Ļ����Ƶ��ף���0mL��
�ۼ���20mL���ᣬ
�ܴ���Ӧ��ֽ��к��������ƶ������»���ǰ�˶�Ӧ�Ŀ̶�ΪV mL
�ش���������
��1�� ����װ�������Եķ����ǣ� ��
��2�����ڲ����������������ֹ۵㣬�۵�һ��������������ΪVmL���۵����������������Ϊ(V-20)mL������Ϊ ����۵�һ�����۵��������ȷ��
��3�������ʵ���ڱ�״���½��У�b=0.5g��V=76mL������Ʒ��̼���Ƶ������ٷֺ���Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ʵ�����У�������̼�����ơ��Ȼ��ơ��Ȼ�淋������ܽ�ȵIJ��죬ͨ������ʳ��ˮ�����Ͷ�����̼��Ӧ�����̼�����ƾ��壬��Ӧԭ���������»�ѧ����ʽ��ʾ��NH3+CO2+NaCl+H2O NH4Cl+NaHCO3�����壩���ݴ�ԭ�������Ƶ�̼���ƾ��壬ijУѧ�����������ʵ��װ�ã�����Bװ���е��Թ��������а����Ȼ��Ƶ���Һ���Ҷ��߾��Ѵﵽ���ͣ�
��1��Aװ������������Ӧ�����ӷ���ʽΪ ��
Cװ����ϡ���������Ϊ ��
��2���±������г�������������ڲ�ͬ�¶��µ��ܽ�����ݣ�g/100gˮ��

���ձ������ݣ������Bװ����ʹ�ñ�ˮ����Ϊ ��
��3����Уѧ���ڼ�������װ�������Ժ����ʵ�飬���û�еõ�̼�����ƾ��壬ָ����ʦָ��Ӧ�� װ��֮�䣨��д��ĸ������һ��ʢ�� ��ϴ��װ�ã��������� ��
��4�����øĽ����װ�ý���ʵ�飬��B�е��Թ��������˾��壬����Ҫ�IJ�����õ���һ�ִ����ľ��塣���������Լ������ᡢŨ��ˮ����ʯ�ҡ�����ˮ�����ñ������ṩ���Լ���ֻ��һ�֣����Թܡ��ƾ��Ƶ���Ҫ������ͨ����ʵ���жϸþ�����̼�����ƾ��壬������̼����炙�ʳ�ξ��壬��������������ʵ�������ۣ�
��
��5������Уѧ������ʵ��ʱ�����ñ���ʳ��ˮ�к�NaCl������Ϊ5.85g��ʵ���õ������NaHCO3���������Ϊ5.04g����NaHCO3�IJ���Ϊ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com