
ijʵ��С�����ñ���ʳ��ˮ�����ߡ�ֱ����Դ���á��� ��
����ʾ�����ձ�����
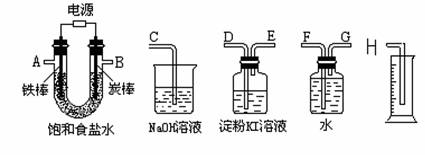
�������ƣ��á�����ʾ���������缫�����ֱ���M��N�����е绯ѧʵ��̽����
��ͬѧ��װ���������Ӻ�ֱ����Դͨ�缸���ӣ�����M����Һ��dz��ɫ����һ��ʱ�䣬��Һ��û��������ֺ���ɫ��
��ͬѧ���õ������ͼ�ͬѧ�Ŀ���ȥ��ͬ�����Ӻ�ֱ����Դͨ�缸���ӣ�ȴ�ŵ�һ�ɴ̱ǵ���ζ������ֹͣͨ�硣
��ͬѧ��װ����������·�պϼ����Ӻ�ȴû�з�������������������ֺܿ�������������ƣ����ֵ����Ƶ�ָ�뷢����ƫת��
���������ͬѧ��ʵ������ش��������⣺
��1��M�缫�������ǣ�д��ѧʽ�� ��N�缫�������ǣ�д��ѧʽ�� ��
��2���������������ɶ�Ӧ����ͬѧ��װ��ͼ��
��3����Ҫ��д������ͬѧʵ��������漰�ķ�Ӧ����ʽ��
��ͬѧN�缫����ʽ
��ͬѧ�ܷ�Ӧ�����ӷ���ʽ
��ͬѧN�缫����ʽ
��4���û�ѧ����ʽ���ͼ�ͬѧʵ��ʱ�۲쵽M����Һ���ֻ��Ǻ�תΪ����ɫ�����ԭ��
��
��5����ͬѧΪ�˱���M�缫������ʴ�������Խ�N�缫������Ϊ��д��ѧʽ�� ��Ϊ��֤�÷���������Ч�����������жԱ�ʵ�飺��ͨ��·2���Ӻֱ���M�缫������2�λ�ɫK3[Fe(CN)6]��Һ������û�и���N�缫�����ձ��е������� ��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �ҹ����������Ҫ�������ڣ�����Ŀǰ��Ҫ��������FeS2��Ϊԭ�������ᣬ�׳�������
�ҹ����������Ҫ�������ڣ�����Ŀǰ��Ҫ��������FeS2��Ϊԭ�������ᣬ�׳�������
| ||
| ||
| V2O5 |
| �� |
| V2O5 |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ȤС����������������װ��һ��װ�ã�������ⱥ��ʳ��ˮ�����û��Һ��pH����֤�����������ԡ�
��1��ʵ��ʱ���������ӿڵ�����˳�����ţ��ǣ�A��__________________________��B��__________________________��
��2��̿����ֱ����Դ��________��������ܷ�Ӧ����ʽ�� ��
��3����֤���������������Ե�ʵ��������________________________________��
��4���ٶ�װ��ı���ʳ��ˮΪ50mL(���ǰ����Һ����仯�ɺ���)������õ�����Ϊ5.6mL����״����ʱֹͣͨ�磬ҡ��U�ι��ڵ���Һ����Һ��pHԼΪ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ҹ����������Ҫ�������ڣ�����Ŀǰ��Ҫ��������FeS2��Ϊԭ�������ᣬ�׳�������
��1��д����������������Ӧ�Ļ�ѧ����ʽ����������������������������������������������
��ҵ�ϣ��÷�Ӧ�����������������������豸���ƣ��н��С�
��2��ij����SiO2������������1�ˣ��������г�����պ�������Ϊ0.76g�����������ʽ���Ĵ���Ϊ����������%
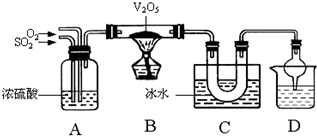
��֪SO3�۵�16.83�桢�е�44.8�档ij��ȤС������SO2��O2�ڴ���V2O5���������Ʊ�SO3���塣װ������ͼ��ʾ
 |
��3��д��B�д���V2O5�����������ķ�Ӧ�Ļ�ѧ����ʽ������������������������������
��4��ѧ��ʵ��ʱ������Cװ��U�ιܵ��ұ��а������ɡ��Ľ��İ취����������������������
��5��װ��D������������β�������и���ܵ�������������������������������������װ��D
����װҺ���Լ�����Ϊ�� ��
A������ʳ��ˮ B���ƾ� C��BaCl2��Һ D��NaOH��Һ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com