
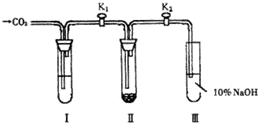
 Ϊ��̽����������̼�Ƿ���ˮ����ʱ���ܺ������Ʒ�Ӧ���������о�С���ͬѧ���������ͼ��ʾ��ʵ��װ�ã��ֱ���мס�������ʵ�飺
Ϊ��̽����������̼�Ƿ���ˮ����ʱ���ܺ������Ʒ�Ӧ���������о�С���ͬѧ���������ͼ��ʾ��ʵ��װ�ã��ֱ���мס�������ʵ�飺


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ��̽����������̼�Ƿ���ˮ����ʱ���ܺ������Ʒ�Ӧ���������о�С���ͬѧ���������ͼ��ʵ��װ�ã��ֱ���мס�������ʵ�飺

ʵ��ף�����Ķ�����̼�������Ƶķ�Ӧ���ڸ�����Թ� �� ��װ��������ƣ���ͨ�������̼ǰ���ر�K1��K2�����Թ�I��װ���Լ�X��K1��K2��ͨ�������̼�������Ӻ������ǵ�ľ�������Թ� �� ��Һ���ϣ��۲쵽ľ������ȼ���� �� �еĵ���ɫû�б仯��
ʵ���ң���ʪ�Ķ�����̼�������Ƶķ�Ӧ�� ���Թ�I��װ���Լ�Y����������ͬʵ��ף��۲쵽ľ����ȼ���� �� �еĵ���ɫ��Ϊ��ɫ��
�Իش��������⣺
��1����װ��������ƺ�ͨ�������̼ǰ����K1��K2 ��Ŀ���� ��
��2����ʵ����У��Լ�X�� ���������� ����ʵ�������Լ�Y�� ��
��3��������������ʵ�����õ��Ľ����� ��
��4���Թܢ��е�����������Һ��������
��5��Ϊ��ȷ��ʵ�������ȷ�ԣ��Ʊ�������̼���õķ�Ӧ�����ѡ�� �����ţ���
A ����ʯ B С�մ� C �ռ� D ���� E ϡ���� F ϡ����
��6��CO2��Na2O2�ķ�Ӧ��������ʾ��ԭ�ӷ�������֤������������з�Ӧ��
Na2O2+ C18O2 +H218O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
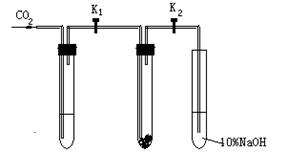
ijУ�����о�С���ͬѧ��Ϊ��̽����������̼��ʲô�����º������Ʒ�Ӧ������ͬѧ��������µ�ʵ��̽��:
ʵ��һ������Ķ�����̼�������Ƶķ�Ӧ:
�ڸ�����Թܢ���װ��Na2O2,�����������Թܵ��Թ������Թ��������������ϵ�K1��K2���ڹر�״̬�������Թܢ���װ���Լ�X��ͼ����װ�ã���K1��K2��ͨ��CO2�������Ӻ������ǵ�ľ�������Թܢ��У��۲쵽ľ������ȼ���Ң��еĵ���ɫû�б仯��
ʵ�������ʪ�Ķ�����̼�������Ƶķ�Ӧ��
���Թܢ���װ���Լ�Y����������ͬʵ��һ���۲쵽ľ����ȼ���Ң��еĵ���ɫ��Ϊ��ɫ��
�� �� ��
�Իش��������⣺
��1����ʵ��һ�У��Լ�X�� �� �������� ��
��2����ʵ����У��Լ�Y�� ��
��3����װ��Na2O2�����������Թ������ҹر�K1��K2��Ŀ����
��
��4���Թܢ��е�NaOH��Һ�������� ��
��5���������������Ա�ʵ�������ͬѧ�õ��Ľ����ǣ�
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣�ijУ�����о�С���ͬѧ��Ϊ��̽����������̼��ʲô�����º������Ʒ�Ӧ������ͬѧ��������µ�ʵ��̽��:
ʵ��һ������Ķ�����̼�������Ƶķ�Ӧ:
�ڸ�����Թܢ���װ��Na2O2,�����������Թܵ��Թ������Թ��������������ϵ�K1��K2���ڹر�״̬�������Թܢ���װ���Լ�X��ͼ����װ�ã���K1��K2��ͨ��CO2�������Ӻ������ǵ�ľ�������Թܢ��У��۲쵽ľ������ȼ���Ң��еĵ���ɫû�б仯��
ʵ�������ʪ�Ķ�����̼�������Ƶķ�Ӧ��
���Թܢ���װ���Լ�Y����������ͬʵ��һ���۲쵽ľ����ȼ���Ң��еĵ���ɫ��Ϊ��ɫ��
�� �� ��
�Իش��������⣺
��1����ʵ��һ�У��Լ�X�� �� �������� ��
��2����ʵ����У��Լ�Y�� ��
��3����װ��Na2O2�����������Թ������ҹر�K1��K2��Ŀ����
��
��4���Թܢ��е�NaOH��Һ�������� ��
��5���������������Ա�ʵ�������ͬѧ�õ��Ľ����ǣ�
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ��һ��ѧ�ڵ����ν��Բ��Ի�ѧ�� ���ͣ�ʵ����
��12�֣�ijУ�����о�С���ͬѧ��Ϊ��̽����������̼��ʲô�����º������Ʒ�Ӧ������ͬѧ��������µ�ʵ��̽��:
ʵ��һ������Ķ�����̼�������Ƶķ�Ӧ:
�ڸ�����Թܢ���װ��Na2O2,�����������Թܵ��Թ������Թ��������������ϵ�K1��K2���ڹر�״̬�������Թܢ���װ���Լ�X��ͼ����װ�ã���K1��K2��ͨ��CO2�������Ӻ������ǵ�ľ�������Թܢ��У��۲쵽ľ������ȼ���Ң��еĵ���ɫû�б仯��
ʵ�������ʪ�Ķ�����̼�������Ƶķ�Ӧ��
���Թܢ���װ���Լ�Y����������ͬʵ��һ���۲쵽ľ����ȼ���Ң��еĵ���ɫ��Ϊ��ɫ��

�� �� ��
�Իش��������⣺
��1����ʵ��һ�У��Լ�X�� �� �������� ��
��2����ʵ����У��Լ�Y�� ��
��3����װ��Na2O2�����������Թ������ҹر�K1��K2��Ŀ����
��
��4���Թܢ��е�NaOH��Һ�������� ��
��5���������������Ա�ʵ�������ͬѧ�õ��Ľ����ǣ�
��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com