
��10�֣���������ʮ�����ʣ���H2 ���� ��Na2O ��CO2 ��H2SO4 ��Ba(OH)2 �ߺ��ɫ�������������� �ఱˮ ��ϡ���� ��Al2(SO4)3
��1�����У����ڻ�������  ��������������� �����ڵ��ʵ��� ������С������������
��������������� �����ڵ��ʵ��� ������С������������
��2������ʮ������������������֮��ɷ������ӷ�Ӧ��H����OH��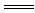 H2O�������ӷ�Ӧ��Ӧ�Ļ�ѧ����ʽΪ ��
H2O�������ӷ�Ӧ��Ӧ�Ļ�ѧ����ʽΪ ��
��3��17.1g�� ����ˮ���250mL��Һ�� SO42-�����ʵ���Ũ��Ϊ ��
 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д� ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д� ��ʦָ��һ��ͨϵ�д�
��ʦָ��һ��ͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ����� | ������ | ����� | �ǵ���� | |||
| ���ڸ�������� | �� | ��� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ����� | ������ | ����� | |||
| ���ڸ�������� | �� | ��� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ����� | ������ | ����� | |||
| ���ڸ�������� | �� | ��� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ����� | ������ | ����� | |||
| ���ڸ�������� | �� | ��� | �� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com