
(17��)ijͬѧ������ͼ��ʾ��ʵ��װ�ý�������ˮ������Ӧ��ʵ��,�������о������仯����IJ������ʡ�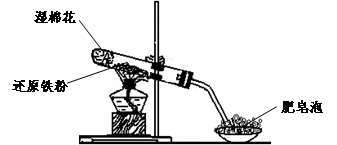
��֪:��FeO + 2H+ = Fe2+ + H2O��Fe2O3 + 6H+ = 2Fe3+ +3 H2O ��Fe3O4 + 8H+ = Fe2+ +2Fe3+ +4 H2O
��ش���������:
��1��Ӳ���Թ��з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2����ͬѧ��ȷ����Ӧһ��ʱ���Ӳ���Թ��й������ʵijɷ�,���������ʵ�鷽��:
�ٴ�Ӳ���Թ���ȴ��,ȡ�������еĹ�����������ϡ�������ҺB;
��ȡ������ҺB�μ�KSCN��Һ,����Һ���ɫ��˵��Ӳ���Թ��й������ʵijɷ���(ֻ��һ��ѡ���������) ,����Һδ���ɫ��˵��Ӳ���Թ��й������ʵijɷ���(ֻ��һ��ѡ���������) ��
| A��һ����Fe3O4,������Fe | B��ֻ��Fe(OH)3 | C��һ����Fe3O4��Fe |
| D��һ����Fe(OH)3,������Fe E.ֻ��Fe3O4 |


��1��3Fe + 4H2O(g)  ��
�� Fe3O4 + 4H2 (2��) ��2��A,C(2��)
Fe3O4 + 4H2 (2��) ��2��A,C(2��)
��3��Fe + 2Fe3+�� 3Fe2+(2��)(���ִ˷���ʽ�Ÿ���,ֻдFe3O4 + 8H+�� Fe2+ +2Fe3+ +4 H2O������)
��4��FeSO4 + 2NaOH �� Fe(OH)2��+ Na2SO4 (2��) 4Fe(OH)2 + O2 + 2H2O �� 4Fe(OH)3 (2��)
��5����ԭ��1�֣�,Fe2+�ױ������е��������������ʣ�1�֣�,���ۣ�1�֣�
(6)������ˮ��У�1�֣����������ۺ�ϡ���ᷴӦ�����������ų��Թ�A��B�еĿ�������ֹFe(OH)2������������������2�֣���B��1�֣���
�������������1��Ӳ���Թ��з�����Ӧ������ˮ�����ķ�Ӧ��ע����ֻ����������������������������д��������������
��2������Һ���ɫ��˵����Һ�������������ӣ������ų���Һ���Ƿ����������ӣ�����Ӳ���Թ��й������ʵijɷ�һ����Fe3O4,������Fe������Һδ���ɫ��˵����Һ�������������ӣ���һ���й�����������������ԭΪ���������ӣ�Ӳ���Թ��й������ʵijɷ�һ����Fe3O4��Fe��
��3��˵��������������������ԭΪ��������Fe + 2Fe3+�� 3Fe2+��
��4�����ɰ�ɫ����������������,Ѹ�ٱ�ɻ���ɫ,����ɺ��ɫ����������������������Ϊ��������, FeSO4 + 2NaOH �� Fe(OH)2��+ Na2SO4�� 4Fe(OH)2 + O2 + 2H2O �� 4Fe(OH)3��
��5��δ������Һ��ɺ�ɫ��Fe2+ ������Ϊ���������ӣ�˵�����л�ԭ�ԡ�ʵ�����к�Fe2+������Һ�����������Ƶ�ԭ�����ױ������е��������������ʣ��������ƺ�Fe2+������ҺʱӦ�����������ۣ�������������������ٻ�ԭ���������ӡ�
��6����ò���O2������ˮ�ķ���һ���������Һ���������塣��Ӧ��ʼʱ����ֹˮ�е�Ŀ�����������ۺ�ϡ���ᷴӦ�����������ų��Թ�A��B�еĿ�������ֹFe(OH)2������������������һ��ʱ��ر�ֹˮ�У�A�е����������ų������Թ���ʹѹǿԽ��Խ��A�е������������뵽B�У���B�л���ְ�ɫ����Fe(OH)2��
���㣺��������ˮ������Ӧ��ʵ�飬������������������ķ������������������Ʊ��������ǵ�ת����
���������������ʼ��仯�����ת���Ǹ��е��ص���ѵ㣬���������ӵļ��顢���ӡ����ʵ��Ʊ������ͳ��֣��Ƚ��ۺϣ�����ѧ�����ۺ�˼ά������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(17��)ijѧ������ѧϰ�С����Խ̲���ͭ��Ũ���ᷴӦ��������о����ܹ���ͭ��Ӧ����������Ũ���Ƕ��٣��������⣬����������·�������ʵ�飺
ʵ���Լ���18mol/L����20mL����ͭ������������2mol/LNaOH��Һ
�����ʵ��ش����⣺
�����ȸ�����ͼ��ʾ����װʵ��װ�ã����ڼ����Լ�ǰ�Ƚ��� ������
���ձ�����NaOH��Һ���յ������ǣ� (�ѧʽ)�����õ��õ�©�������ǽ�������ֱ�������ձ��е�Ŀ���ǣ� ��
�Ǽ�����ƿ20���ӣ���ƿ�з�����Ӧ�Ļ�ѧ����ʽ�ǣ� ������ƿ�з�Ӧ������������ȥ�ƾ��ƣ�������ƿ�е�����ʹ��Ӧ������ȫ��Ȼ���ɵ���aͨ�������Ŀ�������ȷ����ƿ�е�SO2����ȫ�������ձ��С��ڸ�ʵ��װ���е�
(����������)����ȷ������������ֲ�������á�
�Ƚ���ַ�Ӧ����ձ�ȡ�£������м����������ữ��˫��ˮ���ټ���������BaCl2��Һ���ٽ��� �� �� ��������ᱵ������Ϊ13.98g�����������ͭ��Ӧ�������Ũ������� ��
���е�ͬѧ�����������п��Բ��ؼ����ữ��˫��ˮ��ֱ�ӽ��к����ʵ�飬Ҳ�ܵõ�ȷ�����ݣ����������������Ƿ���Ҫ����˫��ˮ��ԭ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡ�����и���12���¿���ѧ�Ծ� ���ͣ�ʵ����
(17��)ʵ����������ƿʧȥ��ǩ���ᣬ�ֱ���Ũ���ᡢŨ�����Ũ���ᡣ
��1����ͬѧ��Ϊ���ý���ͭ���Լ��ɼ������������ᣬ���û�ѧ����ʽ�ͱ�Ҫ�����ּ���˵����____________________________________________________________________��
��2�������һ��ʵ��װ�ã�ʹͭ��ϡ���ᷴӦ��������ͭ��������������ķ����ڻ���װ��ͼ���������缫�������ƺ͵������Һ���ơ�
��3��ʵ����������Ũ��������2.0mol��L-1��ϡ����500mL��
��ʵ���������������������Ͳ���ձ����������⣬����Ҫ��������____________________��
�����в��������������ҺŨ��ƫ�͵���_____________��
a������Ͳ��ȡŨ����ʱ���ӿ̶���
b������ʱ���ӿ̶���
c��ת����Һ��δϴ���ձ��Ͳ�������ֱ�Ӷ���
d�����ݺ�ҡ�ȣ�����Һ����ڿ̶ȣ�δ��������ˮ���̶�
��4����ͼ��ʾΪʵ����ģ�ҵ�����ð����������Ʊ������ʵ��
��װ��A�Ʊ����ﰱ����װ��B�Ʊ�����������
��װ��A���Թ��з�����Ӧ�Ļ�ѧ����ʽΪ_______________________________��
װ��B����ƿ�ڷ�����Ӧ�Ļ�ѧ����ʽΪ__________________________________��
�����й���A��Bװ�õ���������ȷ����__________
a����ѡ����ʵ��Լ�����Bװ��Ҳ���Ʊ�����
b��ʵ������У�A��Bװ����һ����һ������������ԭ��Ӧ
c��U�ι��е��Լ�������ͬ�������ò���ͬ
�۰���a��c��b��d��˳������װ�ý���ʵ�顣
�������ڷ�����Ӧ�Ļ�ѧ����ʽΪ___________________________________��
ʵ�������ijͬѧ���װ��C���Թ���������Һ��pH��7���ó��Ľ���Ϊ����Һһ�������ᡣ�ý���__________������ܡ������ܡ��������������_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�������ʡ�����е���У������һ�θ߿�ģ�����������ۣ���ѧ���� ���ͣ�ʵ����
(17��)ijѧ������ѧϰ�С����Խ̲���ͭ��Ũ���ᷴӦ��������о����ܹ���ͭ��Ӧ����������Ũ���Ƕ��٣��������⣬����������·�������ʵ�飺
ʵ���Լ���18mol/L����20mL����ͭ������������2mol/LNaOH��Һ
�����ʵ��ش����⣺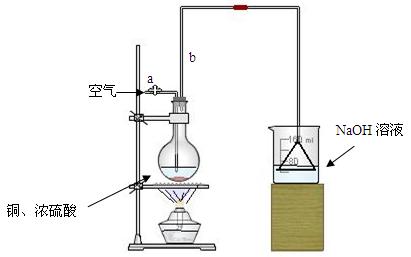
�����ȸ�����ͼ��ʾ����װʵ��װ�ã����ڼ����Լ�ǰ�Ƚ��� ������
���ձ�����NaOH��Һ���յ������ǣ� (�ѧʽ)�����õ��õ�©�������ǽ�������ֱ�������ձ��е�Ŀ���ǣ� ��
�Ǽ�����ƿ20���ӣ���ƿ�з�����Ӧ�Ļ�ѧ����ʽ�ǣ� ������ƿ�з�Ӧ������������ȥ�ƾ��ƣ�������ƿ�е�����ʹ��Ӧ������ȫ��Ȼ���ɵ���aͨ�������Ŀ�������ȷ����ƿ�е�SO2����ȫ�������ձ��С��ڸ�ʵ��װ���е�
(����������)����ȷ������������ֲ�������á�
�Ƚ���ַ�Ӧ����ձ�ȡ�£������м����������ữ��˫��ˮ���ټ���������BaCl2��Һ���ٽ��� �� �� ��������ᱵ������Ϊ13.98g�����������ͭ��Ӧ�������Ũ������� ��
���е�ͬѧ�����������п��Բ��ؼ����ữ��˫��ˮ��ֱ�ӽ��к����ʵ�飬Ҳ�ܵõ�ȷ�����ݣ����������������Ƿ���Ҫ����˫��ˮ��ԭ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015��ɽ��ʡ�����и�һ��ѧ����ĩģ�⻯ѧ�Ծ��������棩 ���ͣ�ʵ����
(17��)ijͬѧ������ͼ��ʾ��ʵ��װ�ý�������ˮ������Ӧ��ʵ��,�������о������仯����IJ������ʡ�

��֪:��FeO + 2H+ = Fe2+ + H2O��Fe2O3 + 6H+ = 2Fe3+ +3 H2O ��Fe3O4 + 8H+ = Fe2+ +2Fe3+ +4 H2O
��ش���������:
��1��Ӳ���Թ��з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2����ͬѧ��ȷ����Ӧһ��ʱ���Ӳ���Թ��й������ʵijɷ�,���������ʵ�鷽��:
�ٴ�Ӳ���Թ���ȴ��,ȡ�������еĹ�����������ϡ�������ҺB;
��ȡ������ҺB�μ�KSCN��Һ,����Һ���ɫ��˵��Ӳ���Թ��й������ʵijɷ���(ֻ��һ��ѡ���������) ,����Һδ���ɫ��˵��Ӳ���Թ��й������ʵijɷ���(ֻ��һ��ѡ���������) ��
A��һ����Fe3O4,������Fe B��ֻ��Fe(OH)3 C��һ����Fe3O4��Fe
D��һ����Fe(OH)3,������Fe E.ֻ��Fe3O4
��3����ͬѧ������ʵ�鷽��������ʵ��,�����Һδ���ɫ,ԭ����
�������ӷ���ʽ��ʾ����
��4����ͬѧ������ȡ������ҺB,ʹ���NaOH��Һ��Ӧ��������ͼ��ʾ�IJ���,�ɹ۲쵽���ɰ�ɫ����,Ѹ�ٱ�ɻ���ɫ,����ɺ��ɫ������,��д��������������صķ�Ӧ�Ļ�ѧ����ʽ ��

��5��һ��ʱ���,��ͬѧ���֣�3����δ������Һ��ɺ�ɫ,˵��Fe2+ ���� �ԡ��ɴ˿�֪,ʵ�����к�Fe2+������Һ�����������Ƶ�ԭ���� ���������ƺ�Fe2+������ҺʱӦ�������� ��
��6����ͬѧΪ�˻�ó־ð�ɫ��Fe(OH)2������������ͼ��ʾװ�ã��ò���O2������ˮ���Ƶ�NaOH��Һ�����Ƶ�FeSO4��Һ��Ӧ����ò���O2������ˮ�ķ�����______________����Ӧ��ʼʱ����ֹˮ�е�Ŀ����___________________________________��һ��ʱ��ر�ֹˮ�У����Թ�_______���A����B�����й۲쵽��ɫ��Fe(OH)2
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com