
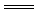 H2O��2�֣�
H2O��2�֣�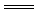 2Fe3++3H2O��2�֣�
2Fe3++3H2O��2�֣�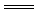 CaCO3��+H2O��2�֣�
CaCO3��+H2O��2�֣�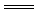 CO2�� + H2O��2�֣�
CO2�� + H2O��2�֣�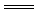 H2O
H2O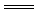 2Fe3++3H2O
2Fe3++3H2O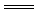 CaCO3��+H2O
CaCO3��+H2O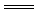 CO2�� + H2O
CO2�� + H2O


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
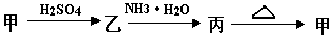
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��̼�ᱵ�����ᷴӦ��BaCO3+2H+==Ba2++CO2��+H2O |
| B����������ϡ���ᷴӦ��2Fe+6H+==2Fe3++3H2�� |
| C������ˮ��Ӧ��2Na+2H2O==2Na++2OH-+H2�� |
| D��NaHCO3��Һ��NaOH��Һ��ϣ�HCO3-+OH-==CO32-+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Na2O2��H2O ��Ӧ�Ʊ�O2��Na2O2 + H2O ="==" 2Na+ + 2OH- + O2�� |
| B��������������Һ��ȥ���������������Ĥ�� Al2O3 + 2OH- ="==" 2AlO2- + H2O |
| C����ʳ�׳�ȥˮƿ�е�ˮ����2CH3COOH + CO32- ="==" 2CH3COO- + CO2��+ H2O |
| D�����������Ũ�ȵ�Ba(OH)2Ũ��Һ��NH4HCO3Ũ��Һ��Ϻ��ȣ�������ͳ���������Ba2+ + 2OH- + NH4+ + HCO3- ="==" BaCO3��+ NH3��+2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������������������������������̼ |
| B���Ȼ����������ƣ����������������� |
| C��̼���������̼��������� |
| D��ʯ��ʯ�����ᷴӦ��ʯ��ʯ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��FeCl3��Һ��Cu�ķ�Ӧ��Cu��Fe3����Cu2����Fe2�� |
| B��NO2��ˮ�ķ�Ӧ��3NO2��H2O��2NO3����2H����NO |
| C��������Һ��ˮ���е�CaCO3��Ӧ��CaCO3��2H����Ca2����H2O��CO2�� |
D���ò��缫����Ȼ�þ��Һ��2Cl-�� 2H2O 2OH���� Cl2��+H2�� 2OH���� Cl2��+H2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A������������KIO3��Һ��KI��Һ������Ӧ����I2��IO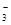 +5I +5I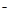 +3H2O=3I2+6OH +3H2O=3I2+6OH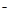 |
B����NaOH��Һ�еμ���ͬ���ʵ���Ũ�ȵ�����Ca(HCO3)2��Һ�� Ca2+ + HCO3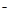 +OH +OH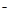 =CaCO3��+H2O =CaCO3��+H2O |
C��NH4HCO3��Һ�����KOHŨ��Һ���ȣ�NH4++ HCO3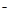 + 2OH�� + 2OH��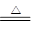 NH3��+ 2H2O + CO32 NH3��+ 2H2O + CO32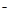 |
| D��ϡ�����������м��Ӧ��3 Fe + 8H+ +2 NO3- =" 3" Fe3+ +2 NO�� + 4 H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ϡ���ᣬNa2CO3��Һ | B��Cu��FeCl3��Һ |
| C��AlCl3��Һ��NaOH��Һ | D������ϡ������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 2�����V���Ĺ�ϵ��ͼ��ͼ��AB�α�ʾ���Ⱥ����ӷ���ʽ�ǣ� ��
2�����V���Ĺ�ϵ��ͼ��ͼ��AB�α�ʾ���Ⱥ����ӷ���ʽ�ǣ� ��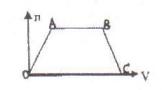
| A���ڢ� | B���ݢ� | C���ܢ� | D���ܢ� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com