
����ʯ��������Ṥҵ��β��(��NO��NO2)��Ӧ�����ܾ���β�������ܻ��Ӧ�ù㷺��Ca(NO2)2���䲿�ֹ����������£�
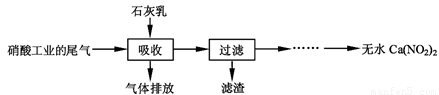
��ش��������⣺
��1��һ�������£�NO��NO2�������з�Ӧ��NO(g)��NO2(g)  N2O3(g)����ƽ�ⳣ������ʽΪK=
��
N2O3(g)����ƽ�ⳣ������ʽΪK=
��
��2�����������в�����Һ�����Ӵ�����(β�����������ײ����룬ʯ�������������������)����Ŀ���� ��������ѭ�����ã���������Ҫ�ɷ��� (�ѧʽ)��
��3���ù��������NO��NO2���ʵ���֮�Ƚӽ�1�U1����n(NO) ��n(NO2)��1�U1,��ᵼ�� ����n(NO) ��n(NO2)��1�U1,��ᵼ�� ��
��4����������Һ�豣�������ԣ���������Һ��Ca(NO2)2�ᷢ���ֽ⣬����֮һ��NO���䷴Ӧ�����ӷ���ʽ ��
��1��k=c(N2O3)/c(NO)��c(NO2) ��2��ʹβ����NO��NO2��������գ�Ca(OH)2��3����������NO�������ߣ���ƷCa(NO2)2��Ca(NO3)2�������ߣ�4��3NO2����2H��=NO3����2NO����H2O
��������
�����������1����ѧƽ�ⳣ������ָ��һ���¶��£����淴Ӧ�ﵽƽ��ʱ��������Ũ�ȵĻ�ѧ���������ݵij˻����Ը���Ӧ��Ũ�ȵĻ�ѧ���������ݵij˻����õı�ֵ��NO��g��+NO2��g��⇌N2O3��g������ƽ�ⳣ������ʽΪk=c(N2O3)/c(NO)��c(NO2)��2��ʹβ����NO��NO2��ʯ�����ֽӴ���NO��NO2��������գ�������Ҫ�ɷ���Ca(OH)2���ʴ�Ϊ��ʹβ����NO��NO2��������գ�Ca(OH)2 ����3����n��NO����n��NO2����1��1����һ�������������ŷ�������NO�������ߣ���n��NO����n��NO2����1��1�����������������������������ʯ���鷴Ӧ����Ca(NO3)2���ʴ�Ϊ���ŷ�������NO�������ߣ���ƷCa(NO2)2��Ca(NO3)2�������ߡ���4����Ӧ����NO2-��H+����������һ���������������ˮ����Ӧ�����ӷ���ʽΪ3NO2-+2H+=NO3-+2NO��+H2O���ʴ�Ϊ��3NO2-+2H+=NO3-+2NO��+H2O��
���㣺��������������ʼ���Ի�����Ӱ�죻��ѧƽ�ⳣ���ĺ���


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| c(N2O3) |
| c(NO)?c(NO2) |
| c(N2O3) |
| c(NO)?c(NO2) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���¿α���������¿���������ѧ�Ծ���A�������������� ���ͣ������
[2012�����վ�]��10�֣�����ʯ��������Ṥҵ��β��(��NO��NO2)��Ӧ�����ܾ���β�������ܻ��Ӧ�ù㷺��Ca(NO2)2���䲿�ֹ����������£�
��1��һ�������£�NO��NO2�������з�Ӧ��NO(g)��NO2(g)  N2O3(g)����ƽ�ⳣ������ʽΪK��________��
N2O3(g)����ƽ�ⳣ������ʽΪK��________��
��2�����������в�������Һ�����Ӵ�����(β�������������룬ʯ�����������������)����Ŀ����_____________ _________��������ѭ��ʹ�ã���������Ҫ�ɷ���__________(�ѧʽ)��
��3���ù��������NO��NO2���ʵ���֮�Ƚӽ�1��1����n(NO)��n(NO2)��1��1����ᵼ��______________________ ___����n(NO)��n(NO2)��1��1����ᵼ��_________________________________��
��4����������Һ�豣�������ԣ���������Һ��Ca(NO2)2�ᷢ���ֽ⣬����֮һ��NO���䷴Ӧ�����ӷ���ʽΪ______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���¿α���������¿���������ѧ�Ծ���A�����������棩 ���ͣ������
[2012�����վ�]��10�֣�����ʯ��������Ṥҵ��β��(��NO��NO2)��Ӧ�����ܾ���β�������ܻ��Ӧ�ù㷺��Ca(NO2)2���䲿�ֹ����������£�

��1��һ�������£�NO��NO2�������з�Ӧ��NO(g)��NO2(g)  N2O3(g)����ƽ�ⳣ������ʽΪK��________��
N2O3(g)����ƽ�ⳣ������ʽΪK��________��
��2�����������в�������Һ�����Ӵ�����(β�������������룬ʯ�����������������)����Ŀ����_____________ _________��������ѭ��ʹ�ã���������Ҫ�ɷ���__________(�ѧʽ)��
��3���ù��������NO��NO2���ʵ���֮�Ƚӽ�1��1����n(NO)��n(NO2)��1��1����ᵼ��______________________ ___����n(NO)��n(NO2)��1��1����ᵼ��_________________________________��
��4����������Һ�豣�������ԣ���������Һ��Ca(NO2)2�ᷢ���ֽ⣬����֮һ��NO���䷴Ӧ�����ӷ���ʽΪ______________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com