
����2��5��̼ԭ�ӵ�ֱ�������е��ȼ���ȵ����ݼ��±���
| �������� | ���� | ���� | ���� | ���� |
| �е�/�� | ��88.6 | ��42.1 | ��0.5 | 36.1 |
| *ȼ����/kJ��mol��1 | 1 560.7 | 2 219.2 | 2 877.6 | 3 535.6 |
B
��������������ɱ������ݿ�֪����̼ԭ�������ӣ������е����ߣ�����ȼ�������������Թ�ϵ���������ɱ������ݴ��жϡ�
�ɱ������ݿ�֪����̼ԭ�������ӣ������е����ߣ��������ڳ��³�ѹ���Ѳ������壬�������ڳ��³�ѹ�¿϶��������壬��A��ȷ���ɱ������ݿ�֪����̼ԭ�������ӣ�����ȼ�������������Թ�ϵ���������ɱ�������B����ȷ���ɱ������ݿ�֪����̼ԭ�������ӣ������е��ȼ���ȶ��������������ɱ�������C��D��ȷ����ѡB��
���㣺�������������ʡ��������ݻ�ȡ�µ���Ϣ����
�������������е��Ѷȵ����⣬���������ǿ�����ض�ѧ�����������ɺ��ܽ����������������������������ѧ���������������ͷ�ɢ˼ά������������֪���ݣ������仯�����ǽ���Ĺؼ���


 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�ϳ����صķ�Ӧ2NH3(g) + CO2(g)��CO(NH2)2(s)(����) + H2O(l) + Q���������У�
��2NH3(g) + CO2(g)��NH4COONH2(s) + Q1��
��NH4COONH2(s)��CO(NH2)2(s) + H2O(l)�CQ2��
����ʾ��ͼ�У�����ȷ��ʾ�ϳ����ع����������仯����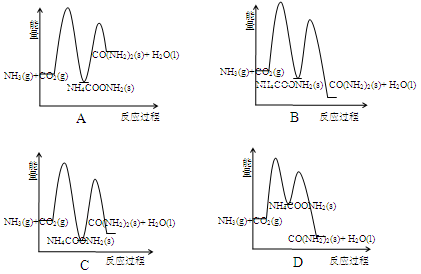
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�ƾ�ȼ�յĹ����У�������������ת���������ж�����ȷ����
| A����ѧ�ܲ���ת��Ϊ���� | B�����ܲ���ת��Ϊ��ѧ�� |
| C�����ܲ���ת��Ϊ��ѧ�� | D�����ܲ���ת��Ϊ��ѧ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���ʯ��ʯī����̼�ĵ��ʣ�ʯī��һ�������¿���ת��Ϊ���ʯ����֪12gʯī��ȫת��Ϊ���ʯʱ��Ҫ����EkJ������������˵����ȷ���ǣ� ��
| A�����ʯ��ʯī��Ϊͬλ�� |
| B��ʯī������ʯ�ȶ� |
| C�����ʯ��ʯī��Ϊͬ���칹�� |
| D����������ʯī����ʯ��ȫȼ�գ����ʯ�ų��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��֪��ӦX��Y=M��NΪ���ȷ�Ӧ���������Ӧ������˵������ȷ����
| A��X������һ������M�ģ�Y������һ������N�� |
| B����Ϊ�÷�ӦΪ���ȷ�Ӧ����һ��Ҫ���ȷ�Ӧ���ܽ��� |
| C���ƻ���Ӧ���еĻ�ѧ�������յ�����С���γ��������л�ѧ�����ų������� |
| D����Ӧ��X��Y��������һ��С��������M��N�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�״����ӽ���Ĥȼ�ϵ���н��״�����ת��Ϊ���������ַ�Ӧԭ����
�� CH3OH(g)+H2O(g)=CO2(g)+3H2(g)����H=��49.0 kJ/mol
�� CH3OH(g)+1/2O2(g)=CO2(g)+2H2(g)����H=��192.9 kJ/mol
����˵����ȷ����
| A��CH3OH��ȼ��Ϊ���ȷ�Ӧ |
B����Ӧ���е������仯��ͼ��ʾ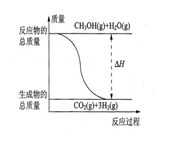 |
| C��CH3OHת���H2�Ĺ���һ��Ҫ�������� |
| D�����ݢ���֪��Ӧ��CH3OH(l)+1/2O2(g)=CO2(g)+2H2(g)�ġ�H >��192.9kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��16�֣�������ҵ�г����ұ�����ķ����Ʊ�����ϩ����֪ij�¶��£�
��Ӧ�٣�CO2(g)+H2(g) CO(g)+H2O(g)����H=" +41.2" kJ/mol��
CO(g)+H2O(g)����H=" +41.2" kJ/mol��
��Ӧ�ڣ� (g)
(g)
 (g)+H2(g)����H=" +117.6" kJ/mol��
(g)+H2(g)����H=" +117.6" kJ/mol��
�١��ڵĻ�ѧ��Ӧƽ�ⳣ���ֱ�ΪK1��K2��
��1����д��������̼�����ұ��Ʊ�����ϩ���Ȼ�ѧ��Ӧ����ʽ ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K= ����K1��K2��ʾ����
��2�����º��������£���Ӧ�ٴﵽƽ���t1ʱ��ͨ������CO2����
����t1֮������淴Ӧ���ߣ���������ע��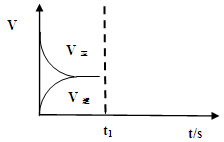
��3����֪ij�¶��£�Ag2SO4������Һ��c(Ag+)="0.04" mol/L������¶�
��Ksp(Ag2SO4)= ����������λ��Ч���֣�
��4����ⷨ�Ʊ��������ƣ�Na2FeO4�����ܷ�ӦʽΪ��Fe+2H2O+2OH-=FeO42-+3H2�����������Һѡ��NaOH��Һ���õ������������� ��д��ѧʽ���������ĵ缫��ӦʽΪ__ _________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��8�֣� ���ڷ�Ӧ����ش���������:
(1)��1L1mol/L��NaOH��Һ�м����������ʣ���ŨH2SO4����ϡ�����ϡ���ᣬǡ����ȫ��Ӧ����ЧӦΪ��H1����H2����H3����������С����˳��Ϊ ��
(2) ʵ���ã���200mL1mol/L��NaOH��Һ�м���ϡ����ǡ�÷�Ӧ�ų�Q kJ����������д���Ȼ�ѧ��Ӧ����ʽ��_________________________ ��
(3)��֪��ӦCH3��CH3(g)�D��CH2=CH2(g)��H2(g)���йػ�ѧ���ļ������¡�
| ��ѧ�� | C��H | C=C | C��C | H��H |
| ����/kJ��mol��1 | 414��4 | 615��3 | 347��4 | 435��3 |
 2NH3(g) ��H=��92��4kJ/mol
2NH3(g) ��H=��92��4kJ/mol�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���ݻ��̶�������ܱ������м���1mol N2��3mol H2������Ӧ��N2(g)+3H2(g) 2NH3(g) ��H=" ��92.4" kJ��mol��1�����н�����ȷ����
2NH3(g) ��H=" ��92.4" kJ��mol��1�����н�����ȷ����
| A���÷�Ӧ�ﵽƽ��ʱ���ų�����������92.4kJ |
| B���ﵽƽ�����������ͨ��1mol������ƽ�ⲻ�ƶ� |
| C�������¶Ⱥ���С�����������ʹ�÷�Ӧ��ƽ�ⳣ������ |
| D�����ﵽƽ��ʱN2��ת����Ϊ20%����ƽ��ʱ�����ڵ�ѹǿ����ʼʱ��90% |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com