
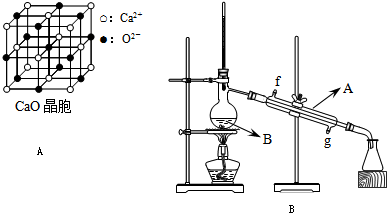
(11��)���ʽṹ��Ԫ���������ǻ�ѧ����Ҫ����֪ʶ��ͨ��ѧϰ�ⲿ��֪ʶ�����Զ���ѧԪ�ػ������֪ʶ�����۽ǶȽ�һ���������⡣��A��B��C��D���ֶ�����Ԫ��,���ǵ�ԭ��������A��D��������,��֪A��Bԭ������ͬ�ĵ��Ӳ���,��A��L���������K�������������, Cȼ��ʱ���ֻ�ɫ����, C�ĵ����ڵ�ȼ��������B�ĵ��ʳ�ַ�Ӧ,���Եõ���D������ɫ��ͬ�ĵ���ɫ��̬������,�Ը����������������������:
(1)Ԫ������:A_________�� B__________�� C__________�� D_________��
(2)д��BԪ�������ڱ��е�λ�ã���______���ڣ���________�塣
(3)A��B���γɶ��ֻ�������л���������ЧӦ����һ�ߵĵ���ʽΪ____________
(4)C�����ڵ�ȼ��������B���ʳ�ַ�Ӧ���ù���Ļ�ѧΪ______________���ù��廯��������Ϊ_______________�����ڵ���������________________��
(5) �õ���ʽ��ʾ������C2D���γɹ���_____________________________________��
 ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||


 ���׳ơ����������������������谷���������ᣨ
���׳ơ����������������������谷���������ᣨ 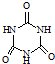 �������������������谷�����֮��ͨ��
�������������������谷�����֮��ͨ��| ������ | �ܶ�/g?cm-3 | �е�/�� | �ܽ��/100gˮ |
| ������ | 0.810 | 118.0 | 9 |
| ������ | 1.049 | 118.1 | �� |
| ���������� | 0.882 | 126.1 | 0.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���㽭ʡ�����и�����ѧ��ʮУ�������п��Ի�ѧ�� ���ͣ������
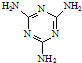
(11��)���ɶ�����Ԫ���γɵij����ǽ������嵥��A�볣����������B���ڼ��������·�Ӧ���ɻ�����C��C��ˮ��Ӧ���ɰ�ɫ����D������E��D��������ǿ�ᣬҲ������ǿ�E������������ȼ�ղ����̼�����ζ����G��G�ڴ������ܵ���������γɡ�E����������������Һ���յõ���ɫ ��ҺF����ҺF�ڿ����г��ڷ��÷�����Ӧ��������֮һΪH��H��һ����Է�������Ϊ78�Ĺ������Ľṹ�ͻ�ѧ�������ƣ�����Һ�Ի�ɫ��
��ҺF����ҺF�ڿ����г��ڷ��÷�����Ӧ��������֮һΪH��H��һ����Է�������Ϊ78�Ĺ������Ľṹ�ͻ�ѧ�������ƣ�����Һ�Ի�ɫ��
��ش��������⣺
(1)��ɵ���A��Ԫ��λ�����ڱ��е�λ�� ��
(2)ָ��H�����еĻ�ѧ������ ��[��Դ:Z+xx+k.Com]
(3)д��B������������Һ��Ӧ�����ӷ���ʽ��______________________��
(4)E������ NaOH��Һ���յõ���Һ�е�����Ũ���ɴ�С��ϵ�� ��
NaOH��Һ���յõ���Һ�е�����Ũ���ɴ�С��ϵ�� ��
(5)G�������������������·�Ӧ����������ɱ�����������ȣ�������2 mol��������ʱ��ת�Ƶ���________mol.
(6)��ҺF�� �����г��ڷ�������H�Ļ�ѧ��Ӧ����ʽΪ�� ��
�����г��ڷ�������H�Ļ�ѧ��Ӧ����ʽΪ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡ����������ѧ��һ��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
(11��)���ʽṹ��Ԫ���������ǻ�ѧ����Ҫ����֪ʶ��ͨ��ѧϰ�ⲿ��֪ʶ�����Զ���ѧԪ�ػ������֪ʶ�����۽ǶȽ�һ���������⡣��A��B��C��D���ֶ�����Ԫ��,���ǵ�ԭ��������A��D��������,��֪A��Bԭ������ͬ�ĵ��Ӳ���,��A��L���������K�������������, Cȼ��ʱ���ֻ�ɫ����, C�ĵ����ڵ�ȼ��������B�ĵ��ʳ�ַ�Ӧ,���Եõ���D������ɫ��ͬ�ĵ���ɫ��̬������,�Ը����������������������:
(1)Ԫ������:A_________�� B__________�� C__________�� D_________��
(2)д��BԪ�������ڱ��е�λ�ã���______���ڣ���________�塣
(3)A��B���γɶ��ֻ�������л���������ЧӦ����һ�ߵĵ���ʽΪ____________
(4)C�����ڵ�ȼ��������B���ʳ�ַ�Ӧ���ù���Ļ�ѧΪ______________���ù��廯��������Ϊ_______________�����ڵ���������________________��
(5) �õ���ʽ��ʾ������C2D���γɹ���_____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��㶫ʡ��ݸ�и߶��ڶ�ѧ����ĩ���Ի�ѧB�� ���ͣ������
(11��)�Ա���Ϊԭ�ϵĺϳ�·��������ʾ���밴Ҫ������

��1��д���������ʵĽṹ��ʽ�� B �� F ��
��2��д����Ӧ�٢ܵĻ�ѧ��Ӧ����ʽ���� ��
�� ��
��3����Ӧ�����õ��Լ��ͷ�Ӧ������ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com