
| ||
| ||
| ||
| ���� |
| ���� |
| ���� |
| 1 |
| 2 |
| 1 |
| 2 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
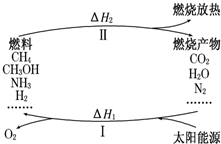
 ����������Ŀǰ�������ٵ�һ���ش���⣮ΪӦ��ȼ��ʹ����ɵĻ�����Ⱦ����ѧ�ҹ���������̫���ܴٽ�ȼ�ϵ�ѭ��ʹ�ã��乹�������ͼ��ʾ��
����������Ŀǰ�������ٵ�һ���ش���⣮ΪӦ��ȼ��ʹ����ɵĻ�����Ⱦ����ѧ�ҹ���������̫���ܴٽ�ȼ�ϵ�ѭ��ʹ�ã��乹�������ͼ��ʾ��
| ||
| ||
| ||
| ���� |
| ���� |
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
(1)ͼ��¬���ɶ��ɺ�����γɵĵ��������ڴ�����CO2�����ľ���������ɺ����(��Ҫ�ɷ���̼���)����ˮ��ʴ����ԭ����(�û�ѧ����ʽ��ʾ) _______________________________��
(2)����ЧӦ���º�ƽ����������������ЧӦ����Ϊԭ����Ҫ�� _____________________��
(3)Ϊ�˿�������ЧӦ��������ѧ������˲��ٷ��������롣���˸���Һ̬CO2�ܶȴ��ں�ˮ�ܶȵ���ʵ�����뽫CO2Һ������������ף��Լ�С������CO2��Ũ�ȡ�ΪʹCO2Һ�����ɲ��õĴ�ʩ��( )
A.��ѹ������ B.��ѹ������
C.��ѹ������ D.��ѹ������
(4)��ѧ�������ڶ�����̼�ġ����ת���������о����ѹ���Ķ�����̼ת��Ϊ��������������ʡ��罫CO2��H2��1��4�ı�����ϣ�ͨ�뷴Ӧ�������ʵ��������·�Ӧ���ɻ��һ����Ҫ����Դ����������»�ѧ����ʽ��CO2+4H2![]() ( )+2H2O
( )+2H2O
����CO2��H2��ϣ���һ����������1��3�ı���������Ӧ������ij����Ҫ�Ļ���ԭ�Ϻ�ˮ���û���ԭ�Ͽ�����( )
A.���� B.ϩ�� C.Ȳ�� D.������
(5)��Ч�ؼ���������CO2�������ӵ���̬ѧ��ʩ��( )
A.ʹ����Ȼ����ȼ�� B.����ȫ���˿�����
C.ֲ�����֣�����ɭ�� D.��������ú��ʯ�͵�ȼ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
63.ͼ��¬���ɶ��ɺ�����γɵĵ��������ڴ�����CO2�����ľ���������ɺ��������Ҫ�ɷ���̼��ƣ�����ˮ��ʴ����ԭ���ǣ��û�ѧ����ʽʾ��������������������������������������������
64.����ЧӦ���º�ƽ����������������ЧӦ����Ϊԭ����Ҫ�ǣ�������
��������������������������������������������������������������������������������������
65.Ϊ�˿�������ЧӦ��������ѧ������˲��ٷ��������롣���˸���Һ̬CO2�ܶȴ��ں�ˮ�ܶȵ���ʵ�����뽫CO2Һ������������ס��Լ�С������CO2��Ũ�ȡ�ΪʹCO2Һ�����ɲ��õĴ�ʩ�ǣ�������
A.��ѹ�����¡���B.��ѹ������ C.��ѹ�����¡���D.��ѹ������
66.��ѧ�������ڶ�����̼�ġ����ת���������о����ѹ���Ķ�����̼ת��Ϊ��������������ʡ��罫CO2��H2��1��4�ı����ºϣ�ͨ�뷴Ӧ�������ʵ��������¼���Ӧ���ɻ��һ����Ҫ����Դ����������»�ѧ����ʽ��CO2+4H2����������+2H2O������CO2��H2��ϣ���һ����������1��3�ı���������Ӧ������ij����Ҫ�Ļ���ԭ�Ϻ�ˮ���û���ԭ�Ͽ����ǣ�������
A.��������B.ϩ�� C.Ȳ������D.������
67.���١���Ч�ؼ���������CO2�������ӵ���̬ѧ��ʩ�ǣ�������
A.ʹ����Ȼ����ȼ��
B.����ȫ���˿�����
C.ֲ�����֣�����ɭ��
D.��������ú��ʯ�͵�ȼ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ����������ʵ����ѧ�߶����ϣ����л�ѧ�Ծ��������棩 ���ͣ������
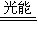 2CO+O2
2CO+O2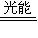 2H2+O2
2H2+O2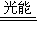 4NH3+3O2
4NH3+3O2 2CH3OH+3O2
2CH3OH+3O2 CH4+ ��
CH4+ �� CH4+
CH4+ 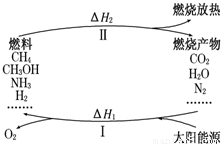
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com