
��12�֣�����AlCl3��FeCl3������к�������FeCl2��Al2��SO4��3��ijͬѧ��Ʋ�����ʵ��Ըû������з��룬�Եõ�������AlCl3��FeCl3��ͼ���Ǹ�ͬѧ��Ʋ����е�ʵ�飺
��1��ʵ���������������˵��Լ�������Ϊ���ѡ�õ��Լ���___________��
A��KMnO4��H������Һ B������ C��H2O2 D��Ũ����
��2������V�����ӷ���ʽΪ_______________________________________________��
�����������ӷ���ʽΪ________________________________________________��
��3��ͼ���Dz�������X��ȡ��ˮFeCl3��AlCl3�IJ���װ�ã�����̨������װ�õ�����ȥ����
��ͼ���У�װ��a��____________��Բ����ƿ��˫�����͵�����ɡ�
��ѭ������A��______________________��
��װ��b���������ʿ�����_______________________�����ţ���
A����ʯ�� B����ˮ�Ȼ��� C��Ũ���� D������������
��4������Ϊ��ͬѧ��ʵ�����_____________����ܡ����ܡ����õ�������AlCl3��FeCl3�������ܣ��������Ƹ�ͬѧ��ʵ�鲽����ƣ���������������________________
_____________________________________________�������ܣ��˿ղ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| OH- |
| H+ |
| OH- |
| H+ |
| ||
| ||
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�꽭��ʡ���и�����ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
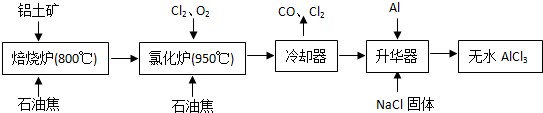
��ˮAlCl3�������л��ϳɵĴ�����ʳƷ���ɼ��ȡ���ҵ������������Ҫ�ɷ���A12O3��Fe2O3����ʯ�ͽ�����Ҫ�ɷ���C������ͼ��ʾ���̽���һϵ�з�Ӧ���Ʊ���ˮAlCl3��

��1���Ȼ����ڼ�������������������̬�Ȼ����Ļ�ѧʽΪAl2Cl6��ÿ��Ԫ�ص�ԭ���������ﵽ8�����ȶ��ṹ����AlCl3�ǣ� ���壬��ṹʽΪ�� ��
��2���Ȼ�¯��Al2O3��Cl2��C��Ӧ�Ļ�ѧ����ʽ�� ��
��3����ȴ���ų���β���к��д���CO������Cl2������Na2SO3��Һ��ȥCl2���˷�Ӧ�����ӷ���ʽΪ�� ��
��4������������Ҫ����AlCl3��FeCl3�����������Al���������ǣ� ��
��5��AlCl3��Ʒ��FeԪ�غ���ֱ��Ӱ����Ʒ�ʣ�Ϊ�ⶨ��Ʒ��FeԪ�صĺ������ֳ�ȡ16.25g��ˮAlCl3��Ʒ�����ڹ�����NaOH��Һ�����˳�����������ᆳϴ�ӡ����ա���ȴ�����ز�����������Ϊ0.32g�����Ʒ��FeԪ�صĺ���Ϊ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ӱ�ʡ����������ģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ������
ij�Ͻ���м����Ҫ�ɷ�ΪCu��Fe��Al��������������ͭ��[Cu2��OH��2CO3]����������������������������������Ͻ���м��ȡ������CuSO4��5H2O������ˮAlCl3������Ĺ�������ͼ��ʾ��
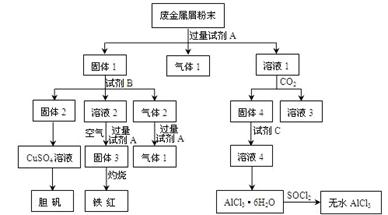
��1���ڷϽ���м��ĩ�м����Լ�A����������1�ķ�Ӧ�����ӷ���ʽ�� ��
��2����Һ2�к��еĽ����������� ������2�ijɷ��� ��
��3����Һ2ת��Ϊ����3�ķ�Ӧ�����ӷ���ʽ�� ��
��4������2��ȡCuSO4��Һ�ж��֡� ���ڹ���2�м���ϡH2SO4��ͨ��O2�����ȣ�ʹ����2ȫ���ܽ��CuSO4��Һ����Ӧ�����ӷ���ʽ�� ��
��5����Һ1ת��Ϊ��Һ4��һϵ�й��̣����ܼ�Ϊ������Һ1��+���Լ�C����Һ4���������� ��
��6��ֱ�Ӽ���AlCl3��6H2O���ܵõ���ˮAlCl3��SOCl2Ϊ��ɫҺ�壬������ˮ��Ӧ����HCl��һ�־���Ư���Ե����塣AlCl3��6H2O��SOCl2��ϼ�����ȡ��ˮAlCl3����Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com