���𰸡�
��������1����Ӧ�е�Ԫ�ػ��ϼ���+5�۽���Ϊ+2��+4�ۣ��������������ԣ�ͬʱ���������Σ������������ԣ�
��2��ͭʧȥ2�������γ�Cu
2+��ʧȥ�ĵ������ʵ�����ͭ��2�����ݴ˼��㣮
���ݵ���ת���غ����NO��NO
2�����ʵ���������ͭԪ���غ��������ͭ�����ʵ��������ݵ�Ԫ���غ��֪�μӷ�Ӧ����������ʵ���n��HNO
3��=2n[Cu��NO
3��
2]+n��NO
2��+n��NO����
���ݷ���ʽ4NO
2+O
2+2H
2O=4HNO
3��4NO+3O
2+2H
2O=4HNO
3��������Ҫͨ��O
2�����ʵ�����������������ʵ���������V=cVm���������������Һ�������һ��������������������֮�ͣ�����c=
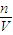
��������Ũ�ȣ�
��3����ͭΪ3mol����HNO
3Ϊ10mol������ͭԪ���غ��֪����������ͭ3mol���������������õ�����Ϊ10mol-6mol=4mol��������NOΪxmol��������NO
2Ϊ��4-x��mol�����õ���ת���غ��з��̼���xֵ���ݴ���д���ӷ���ʽ��
��4���÷�Ӧ��������������ԭ��Ӧ�����ߵı����������������������Է����仯��
����⣺��1����Ӧ�е�Ԫ�ػ��ϼ���+5�۽���Ϊ+2��+4�ۣ��������������ԣ�ͬʱ���������Σ������������ԣ�
�ʴ�Ϊ�������ԡ����ԣ�
��2��ͭʧȥ2�������γ�Cu
2+��ʧȥ�ĵ������ʵ�����ͭ��2����0.004molCu��������ȫ�ܽ��
Cuʧȥ�ĵ�������0.004mol×2×N
Amol
-1=0.008N
A��
����ͭԪ���غ㣬n[Cu��NO
3��
2]=n��Cu��=0.004mol����NO��NO
2�����ʵ���Ϊymol��
���ݵ���ת���غ���3y+y=0.004×2�����y=0.002�����ݵ�Ԫ���غ��֪���μӷ�Ӧ����������ʵ���
n��HNO
3��=2n[Cu��NO
3��
2]+n��NO
2��+n��NO��=0.004mol×2+0.002mol+0.002mol=0.012mol��
���ݷ���ʽ4NO
2+O
2+2H
2O=4HNO
3��4NO+3O
2+2H
2O=4HNO
3��֪����Ҫͨ��O
2�����ʵ���Ϊ
0.002mol×

+0.002mol×

=0.002mol��������������ʵ���Ϊ0.002mol+0.002mol=0.004mol��������Ҫ���������Ϊ
0.002mol×22.4L/mol=0.0448L=44.8ml����Һ�������һ��������������������֮�ͣ�������Һ�����Ϊ
0.004mol×22.4L/mol=0.004×22.4L��������Һ�����ʵ���Ũ����

=

mol/L��
�ʴ�Ϊ��0.008N
A��0.012mol��44.8mL��

mol/L��
��3����ͭΪ3mol����HNO
3Ϊ10mol������ͭԪ���غ��֪����������ͭ3mol���������������õ�����Ϊ10mol-6mol=4mol��������NOΪxmol��������NO
2Ϊ��4-x��mol�����ݵ���ת���غ��֪��3x+��4-x��×1=3×2�����x=1��������NOΪ1mol��������NO
2Ϊ3mol����Ӧ���ӷ���ʽΪ3Cu+10H
++4NO
3-=3Cu
2++NO��+3NO
2��+5H
2O��
�ʴ�Ϊ��3Cu+10H
++4NO
3-=3Cu
2++NO��+3NO
2��+5H
2O��
��4���÷�Ӧ��������������ԭ��Ӧ�����ߵı����������������������Է����仯��
�ʴ�Ϊ���÷�Ӧ��������������ԭ��Ӧ�����ߵı����������������������Է����仯��
���������鷴Ӧ����ʽ���йؼ��㣬�Ѷ��еȣ���3�������ӷ���ʽ����д�ؼ���NO��NO
2�����ʵ�����ȷ����

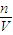 ��������Ũ�ȣ�
��������Ũ�ȣ� +0.002mol×
+0.002mol× =0.002mol��������������ʵ���Ϊ0.002mol+0.002mol=0.004mol��������Ҫ���������Ϊ
=0.002mol��������������ʵ���Ϊ0.002mol+0.002mol=0.004mol��������Ҫ���������Ϊ =
= mol/L��
mol/L�� mol/L��
mol/L��

 ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д� ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д� ����ϰ�⽭��ϵ�д�
����ϰ�⽭��ϵ�д� ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�

 4NO+6H2O
4NO+6H2O 4NO+6H2O
4NO+6H2O +HNO3��Ũ��
+HNO3��Ũ�� -NO2+H2O
-NO2+H2O +HNO3��Ũ��
+HNO3��Ũ�� -NO2+H2O
-NO2+H2O