
����Ŀ��ij��ѧ��ȤС��������ͼװ�ý������������ϳɺͷ����ʵ��̽������ش���������

��1��д���ϳ����������Ļ�ѧ����ʽ_______________________________��
��2������b������________��ͼ���������������õ���______����ᡢ�⡢�㡢�䡢e����
��3��Ϊ��������������IJ��ʿɲ�ȡ�Ĵ�ʩ _______________________________
��4������0.5h���ȷ�Ӧ����Ӧװ��c�дֲ�Ʒת����d�н�������
���� | 98.3%Ũ���� | �������� | ���� | �Ҵ� | ���� | ˮ |
�е� | 338�棬 | 77.1�� | 118�� | 78.5�� | 34.6�� | 100�� |
�����ϱ������������õ������������У����п��ܺ���________________���ʡ�
���𰸡� CH3COOH��CH3CH2OH![]() CH3COOCH2CH3��H2O ������ �� ��CH3COOCH2CH3��ʱ������룻��Ӧ�¶Ȳ��˹��ߣ�����CH3COOH ��CH3CH2OH�Ļӷ�����Ӧ�������ˮ������Ũ�������ˮ����������ƽ��������Ӧ�����ƶ�����߲��ʡ���д������һ�����ɣ� CH3CH2OH
CH3COOCH2CH3��H2O ������ �� ��CH3COOCH2CH3��ʱ������룻��Ӧ�¶Ȳ��˹��ߣ�����CH3COOH ��CH3CH2OH�Ļӷ�����Ӧ�������ˮ������Ũ�������ˮ����������ƽ��������Ӧ�����ƶ�����߲��ʡ���д������һ�����ɣ� CH3CH2OH
����������1���ϳ����������Ļ�ѧ����ʽΪCH3COOH��CH3CH2OH![]() CH3COOCH2CH3��H2O����2������b�����������ܣ�a��ֱ�������ܣ�ͼ���������������õ���a����3������������Ӧ�ǿ��淴Ӧ��������������IJ��ʿɲ�ȡ�Ĵ�ʩΪ����CH3COOCH2CH3��ʱ������룻��Ӧ�¶Ȳ��˹��ߣ�����CH3COOH CH3CH2OH�Ļӷ�����Ӧ�������ˮ������Ũ�������ˮ����������ƽ��������Ӧ�����ƶ�����߲��ʣ���4���ɱ����е����ݿ�֪���Ҵ������������ķе�ӽ����������õ������������У����п��ܺ���CH3CH2OH ���ʡ�
CH3COOCH2CH3��H2O����2������b�����������ܣ�a��ֱ�������ܣ�ͼ���������������õ���a����3������������Ӧ�ǿ��淴Ӧ��������������IJ��ʿɲ�ȡ�Ĵ�ʩΪ����CH3COOCH2CH3��ʱ������룻��Ӧ�¶Ȳ��˹��ߣ�����CH3COOH CH3CH2OH�Ļӷ�����Ӧ�������ˮ������Ũ�������ˮ����������ƽ��������Ӧ�����ƶ�����߲��ʣ���4���ɱ����е����ݿ�֪���Ҵ������������ķе�ӽ����������õ������������У����п��ܺ���CH3CH2OH ���ʡ�


 ��������ѧ����ϵ�д�
��������ѧ����ϵ�д� ��Ԫ������ĩ��ϵ�д�
��Ԫ������ĩ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ʵķ���������ǣ� ��
A. ���������CO2��SiO2��SO2��NO2
B. �NaOH��KOH��Ba(OH)2������
C. �������� Ư�� ˮú�� ������������
D. ���������Na2O2��CaO��MgO��Al2O3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij������ӣ���к���һ���ǻ���(��M��ʾ)��M��̼���ṹ��֧��������ʽΪC4H6O5��1.34 g M��������̼��������Һ��Ӧ�����ɱ�״���µ�����0.448 L��M��һ�������¿ɷ�������ת����M![]() A
A![]() B
B![]() C(M��A��B��C������̼ԭ����Ŀ��ͬ)�������й�˵���в���ȷ����(����)
C(M��A��B��C������̼ԭ����Ŀ��ͬ)�������й�˵���в���ȷ����(����)
A��M�Ľṹ��ʽΪHOOC��CHOH��CH2��COOH
B��B�ķ���ʽΪC4H4O4Br2
C����M�Ĺ��������ࡢ������ȫ��ͬ��ͬ���칹�廹��1��
D��C���ʲ���������ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ����Ҫ����480mL 0.2 mol��L��1 NaCl��Һ��ʵ����ֻ�к������������Ƶ��Ȼ�����Ʒ��������·�������ش��������⣺

���������ᴿ
��1������A�ijɷ�Ϊ________________���ѧʽ����
��2���Լ�2�Ļ�ѧʽΪ_____________���ж��Լ�2�Ƿ�����ķ���_______________������2������__________________��
����������Һ
��1����������ƽ��ȡ�Ȼ��ƣ�������Ϊ________g��
��2�����ƹ�������Ҫʹ�õ�ʵ����������������ƽ��ҩ�ס���Ͳ�����������ձ�����ͷ�ι��⣬����Ҫ___________��
��3��������Ҫ�����������ȷ˳���Ǣ���____��_____�� ____���ܣ�����ţ���
�ٳ�ȡһ���������Ȼ��ƣ������ձ��У�����������ˮ�ܽ�
�ڼ�ˮ��Һ��������ƿ���̶�����1~2����ʱ�����ý�ͷ�ιܵμ�����ˮ����Һ����̶�������
�۽���Һת�Ƶ�����ƿ��
�ܸǺ�ƿ�����������µߵ���ҡ��
��������������ˮϴ���ձ��ڱںͲ�����2��3�Σ�ϴ��Һת�Ƶ�����ƿ��
��4����������������Ƶ�NaCl��ҺŨ���к�Ӱ�죿��ѡ����ƫ��������ƫС��������Ӱ������
������ƿ������ϴ�Ӻ������������ˮ________________��
�ڶ���ʱ����������ƿ�Ŀ̶���________________��
��ת����Һʱ�����������¶˿�������ƿ�Ŀ̶������ϵ��ڱ�________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������(ClNO)���л��ϳ��е���Ҫ�Լ�����ҵ�Ͽ���NO��Cl2�ϳɣ��ش��������⣺
(1)���������£����������������ڴ����еĺ������������ʱ�������������ȣ��漰�Ȼ�ѧ����ʽ��ƽ�ⳣ�����±���
��� | �Ȼ�ѧ����ʽ | ƽ�ⳣ�� |
�� | 2NO2(g) +NaCl(s) | K1 |
�� | 4NO2(g) +2NaCl(s) | k2 |
�� | 2NO(g)+Cl2(g) | K3 |
K3=_______(��K1��K2��ʾ)��
(2)25��ʱ�������Ϊ2L�Ҵ���ѹ�Ƶĺ����ܱ�������ͨ��0.08molNO��0.04molCl2������Ӧ��2NO(g)+Cl2(g) ![]() 2ClNO(g) ��H3��
2ClNO(g) ��H3��
�� ����������˵���÷�Ӧ�Ѵﵽƽ��״̬����_____������ţ�
a.v��(Cl2)=2v��(NO) b.�����ڻ��������ܶȱ��ֲ���
c.����������ѹǿ���ֲ��� d.�����ڻ�������ƽ����Է����������ֲ���
������Ӧ��ʼ��ƽ��ʱ�¶���ͬ����÷�Ӧ������ѹǿ��P)��ʱ�䣨t)�ı仯��ͼI����a��ʾ�����H3______0(� >������ < ����ȷ��"����������������ͬ�����ı�ijһ����ʱ�������ѹǿ(P)��ʱ�䣨t)�ı仯��ͼI����b��ʾ���t�ı��������_________��
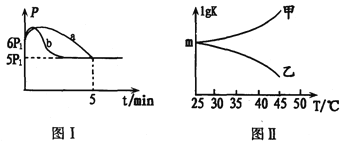
��ͼII�Ǽס���ͬѧ���������Ӧƽ�ⳣ���Ķ���ֵ��lgK)���¶ȵı仯��ϵ��������ȷ��������____(��ס����ҡ�����mֵΪ_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͬһ���ڵ�X��Y��Z����Ԫ�أ���֪��������������Ӧˮ���������ǿ����H3XO4��H2YO4��HZO4�������ƶϲ���ȷ����( )
A. ԭ��������X��Y��Z B. ��̬�⻯���ȶ��ԣ�XH3��H2Y��HZ
C. Ԫ�صķǽ�����ǿ����X��Y��Z D. ������������Z��Y��X
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ϩ���������(M)��������̹����еĿ���ʴͿ�㣬��ϳ�·�����£�

��֪��R1��CHO��R2��CH2CHO![]() CHOHR1CHCHOR2
CHOHR1CHCHOR2
��ش�
(1)C�Ļ�ѧ����Ϊ______________��M�к��������ŵ�����Ϊ____________��
(2)F��G�ķ�Ӧ����Ϊ__________��
�������G�������ɹ����ŵ�ʵ�鷽��Ϊ______________________________��
(3)C��D�Ļ�ѧ����ʽΪ________________________________________��
(4)E�Ľṹ��ʽΪ_______________________��
H��˳ʽ�ṹ��ʽΪ_____________________________��
(5)ͬʱ��������������F��ͬ���칹����________��(�����������칹)��
�����ڷ����廯������ܷ���ˮ�ⷴӦ��������Ӧ
���к˴Ź���������4�����շ�����ʵĽṹ��ʽΪ_________________(��дһ��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���Cl2����ͨ��ˮ�������ͣ�Ȼ���ٵμ�0.1 mol��L��1��NaOH��Һ��������������Һ��pH�仯������ͼ��ʾ������ѡ����ȷ����(����)

A. a����ʾ����Һ��c(H��)��c(Cl��)��c(HClO)��c(OH��)
B. b����ʾ����Һ��c(H��)��c(Cl��)��c(HClO)��c(ClO��)
C. c����ʾ����Һ��c(Na��)��c(HClO)��c(ClO��)
D. d����ʾ����Һ��c(Na��)��c(ClO��)��c(Cl��)��c(HClO)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ɫ���� A ��һ�ֻ���ɫ���� B �п�������ȼ�գ�������ɫ���棬��Ӧ�������� C��B ����� D ��Ӧ�����ɰ�ɫ���� E��D�ڿ�����ȼ������dz��ɫ���� F�� F �� CO2 ��Ӧ�ɵõ����� G��D ��ˮ��Ӧ������ A, A ��G��ȼ������ˮ���ƶϳ��������ʺش��������⣺
��1��д���������ʵĻ�ѧʽ��B _______��C_______��E_______��
��2��д�����л�ѧ����ʽ��
a���� D ���� F��________________________________________
b�� F �� CO2 ��Ӧ��_______________________________________
c��D ��ˮ��Ӧ��________________________________________
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com