
| ��ѧʽ | CH3COOH | H2CO3 | HClO | |
| ����ƽ�ⳣ�� | Ka=1.8��10-5 | Kal=4.3��10-7 | Ka2=5.6��10-11 | Ka=3.0��10-8 |
���� ��1����ĵ��볣��Խ�������Ծ�Խǿ������Խǿ���γ�ǿ�������ε�ˮ��̶Ⱦ�ԽС������ͬŨ���µ�pH��ԽС��
��2�������£�0.1mol•L-1CH3COOH��Һ��ˮϡ�ͣ�c��H+����С��c��OH-�����������¶Ȳ��䣬���볣�����䣬ˮ�����ӻ����䣻
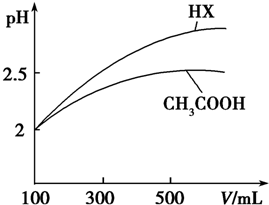
��3�������Ϊ100mL pH=2��CH3COOH��һԪ��HX����Ϊϡ����ͬ������һԪ��HX��pH�仯��CH3COOH�Ĵ�HX���Խ�ǿ��
��4��25��ʱ��CH3COOH��CH3COONa�Ļ����Һ�������pH=6�����ݵ���غ���Եõ�c��CH3COO-��+c��OH-��=c��H+��+c��Na+������c��CH3COO-��-c��Na+��=c��H+��-c��OH-����
��5���ٱ�״���£�1.12L CO2Ϊ0.05mol��100mL 1mol•L-1��NaOH��Һ����NaOH�����ʵ���Ϊ0.1mol�����Եõ�������Ϊ0.05mol•L-1��̼������Һ�����������غ��жϣ�
�ڸ��ݵ���غ������
��� �⣺��1����ĵ��볣��Խ�������Ծ�Խǿ������Խǿ���γ�ǿ�������ε�ˮ��̶Ⱦ�ԽС������ͬŨ���µ�pH��ԽС���ɱ����Կ��������볣��Ϊ�������̼���һ��������ڴ��������̼��Ķ������룬�������ʵ���Ũ�Ⱦ�Ϊ0.1mol•L-1��a��CH3COONa��b��Na2CO3��c��NaClO��d��NaHCO3pH��С�������е�˳����a��d��c��b��
�ʴ�Ϊ��a��d��c��b��
��2��A�������£�0.1mol•L-1CH3COOH��Һ��ˮϡ�ͣ�c��H+����С����A����
B�����ڴ���Ϊ���ᣬ����뷽��ʽΪCH3COOH?CH3COO-+H+������볣��K=$\frac{c��C{H}_{3}CO{O}^{-}����c��{H}^{+}��}{c��C{H}_{3}COOH��}$����Ϊ��ˮ������c��CH3COO-����С���¶Ȳ��䣬K���䣬����$\frac{c��{H}^{+}��}{c��C{H}_{3}COOH��}$ ���B��ȷ��
C���¶Ȳ��䣬ˮ�����ӻ����䣬��c��H+��•c��OH-�����䣬��C����
D����ˮϡ�ͣ�c��H+����С��c��OH-����������$\frac{c��O{H}^{-}��}{c��{H}^{+}��}$ ����D��ȷ��
E���¶Ȳ��䣬K���䣬��$\frac{c��{H}^{+}��•c��C{H}_{3}CO{O}^{-}��}{c��C{H}_{3}COOH��}$���䣬��E����
�ʴ�Ϊ��BD��
��3�������Ϊ100mL pH=2��CH3COOH��һԪ��HX����ˮϡ������pH����Һ����Ĺ�ϵ��ͼ��ʾ����HX�ĵ���ƽ�ⳣ������CH3COOH�ĵ���ƽ�ⳣ������Ϊϡ����ͬ������һԪ��HX��pH�仯��CH3COOH�Ĵ�HX���Խ�ǿ������ƽ�ⳣ���ϴ�
�ʴ�Ϊ�����ڣ�ϡ����ͬ������һԪ��HX��pH�仯��CH3COOH�Ĵ�HX���Խ�ǿ������ƽ�ⳣ���ϴ�
��4��25��ʱ��CH3COOH��CH3COONa�Ļ����Һ�������pH=6�����ݵ���غ���Եõ�c��CH3COO-��+c��OH-��=c��H+��+c��Na+��������c��CH3COO-��-c��Na+��=c��H+��-c��OH-��=10-6-10-8=9.9��10-7mol/L��
�ʴ�Ϊ��9.9��10-7��
��5���ٱ�״���£�1.12L CO2Ϊ0.05mol��100mL 1mol•L-1��NaOH��Һ����NaOH�����ʵ���Ϊ0.1mol�����Եõ�������Ϊ0.05mol•L-1��̼������Һ���ʸ��������غ����֪����c ��OH-��=2c��H2CO3��+c��HCO3-��+c��H+����
�ʴ�Ϊ��c��HCO3-��+c��H+����
�ڸ��ݵ���غ���Եõ���c��H+��+c��Na+��=c��HCO3-��+c��CO32-��+c��OH-����
�ʴ�Ϊ��c��HCO3-��+c��CO32-��+c��OH-����
���� ���⿼������Һ���������ҺpH�Ĺ�ϵ��������ʵĵ��롢�ε�ˮ��ԭ������Ӱ���֪ʶ����Ŀ�Ѷ��еȣ���ȷ�ε�ˮ��ԭ����������ʵĵ���ƽ�⼰��Ӱ������Ϊ���ؼ���ע��������Һ�е��غ��ϵ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
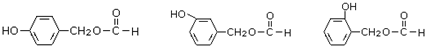
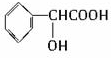
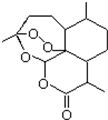 2015��ŵ�������������������ȡ�Ŀ�ű��ҩ�����صĽṹ��ʽ���ü���ʽ��ʾ��ͼ��
2015��ŵ�������������������ȡ�Ŀ�ű��ҩ�����صĽṹ��ʽ���ü���ʽ��ʾ��ͼ�� ����Ҫ���л��ϳ��������ǻ��������ǻ��ķ�Ӧ�����Т٢ڢݣ���ѡ���ţ�
����Ҫ���л��ϳ��������ǻ��������ǻ��ķ�Ӧ�����Т٢ڢݣ���ѡ���ţ�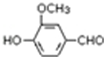 �������ںϳ������أ��ϳ���Ȼ���ȩ�ķ�Ӧ���£�
�������ںϳ������أ��ϳ���Ȼ���ȩ�ķ�Ӧ���£�
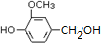 ��
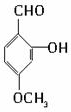
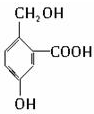
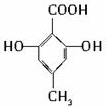
�� ������֮һ����
������֮һ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
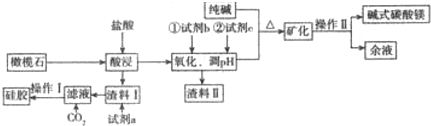
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ��ˮ�루NH4��2SO4��Һ��Ϻ�pH=7����Һ�У�[NH4+]��[SO42-] | |
| B�� | ��ͬ�¶��£�0.2 mol•L-1������Һ��0.1 mol•L-1������Һ�У�[H+]֮�� | |
| C�� | Na2CO3��Һ�У�[Na+]��[CO32-] | |
| D�� | pH=12��Ba��OH��2��Һ��pH=12��KOH��Һ�У�c[Ba��OH��2]��c��KOH�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��10NA������ת��ʱ���ų�1300kJ������ | |
| B�� | ��1NA��ˮ����������ΪҺ��ʱ������1300kJ������ | |
| C�� | ��2NA��̼�����õ��Ӷ�����ʱ���ų�1300kJ������ | |
| D�� | ��8NA��̼�����õ��Ӷ�����ʱ������1300kJ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com