
��֪������A�ǻ���ɫ���壬C�ǵ���ɫ���壬EΪ���ɫ���塣�ں��ʵķ�Ӧ�� ���£����ǿ�����ͼ���з�Ӧ��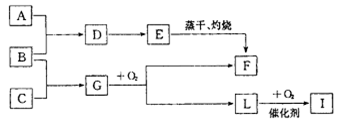
(1) ��ͼF���ʵĻ�ѧʽ________
(2) ��Ҫд����D�Ʊ�E�����ʵ���������_______��
(3) д��B+ C��G��һ�������·����Ļ�ѧ��Ӧ����ʽ_______
(4) д��B�ڳ�ʪ�Ŀ����з����绯ѧ��ʴʱ�������ĵ缫��Ӧʽ��_______
(5) ��һ���¶��£�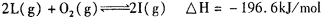 ����һ���ݻ������ �����У�����2mol L��1molO2,ʹ֮��ַ�Ӧ���ų�������ΪQ����Q_______196. 6kJ (� >������������=������
����һ���ݻ������ �����У�����2mol L��1molO2,ʹ֮��ַ�Ӧ���ų�������ΪQ����Q_______196. 6kJ (� >������������=������
��8�֣� ��1�� Fe2O3 (1��) ��
��2�� ��FeCl3������Һ��μ��뵽��ˮ�У����ֱ����ɫ��Ϊ���ɫ��2�֣���
��3��Fe+S FeS��2�֣���
FeS��2�֣���
��4�� 2H2O+O2+4e- =4OH-��2�֣�����5��< ��1�֣�
���������������������Ϣ������A�ǻ���ɫ���壬��AΪCl2��C�ǵ���ɫ���壬��CΪNa2O2��S��EΪ���ɫ���壬��EΪFe(OH)3��BΪFe��CΪS��DΪFeCl3��GΪFeS��FΪ��Fe2O3��LΪSO2��IΪSO3���������Ϸ�����
(1) F���ʵĻ�ѧʽΪFe2O3��
(2) ��D�Ʊ�E�����ʵ����������ǽ�FeCl3������Һ��μ��뵽��ˮ�У����ֱ����ɫ��Ϊ���ɫ��
(3) B+ C��G��һ�������·����Ļ�ѧ��Ӧ����ʽΪFe+S FeS��
FeS��
(4) Fe��ʪ�Ŀ����з����绯ѧ��ʴ��������ʴ���Ǹ�����������O2�������ĵ缫��ӦʽΪ2H2O+O2+4e- =4OH-��
(5)��ӦΪ���淴Ӧ�����ܽ��е��ף���Q<196. 6kJ��
���㣺������ƶ� �����Ļ�ѧ���� ��������Ԫ�صĵ��ʼ��仯������ۺ�Ӧ��
���������⿼��������ƶϣ���Ŀ�Ѷ��еȣ�����ע���ƶ�FeΪ������Ĺؼ����ӻ��ϼ۱仯�ĽǶȷ�����


 �ظ���ʦ�㲦ϵ�д�
�ظ���ʦ�㲦ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
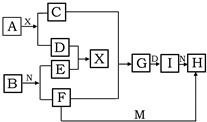 ��������ѧ��ѧ�г������ʵ�ת����ϵͼ�У���֪������AΪ����ɫ���廯���BΪ�������ʣ�D��E��M�dz������嵥�ʣ�����IΪ���ɫ���壬MΪ����ɫ��N��θ�����Ҫ�ɷ֣���ҵ����E��M����ȡN�����ƶϣ�
��������ѧ��ѧ�г������ʵ�ת����ϵͼ�У���֪������AΪ����ɫ���廯���BΪ�������ʣ�D��E��M�dz������嵥�ʣ�����IΪ���ɫ���壬MΪ����ɫ��N��θ�����Ҫ�ɷ֣���ҵ����E��M����ȡN�����ƶϣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
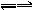
(1)д��F���ʵĻ�ѧʽ������������������?
(2)��Ҫд����D�Ʊ�E�����ʵ��������̣��������������������������������������������������������Ƹý���IJ���������������������?
(3)д��G��O2��һ�������·����Ļ�ѧ��Ӧ����ʽ��?������������������������?��
(4)д��B�ڳ�ʪ�Ŀ����з����绯ѧ��ʴʱ�������ĵ缫��Ӧʽ������������������������(5)��һ���¶��£�2H(g)+O2![]() 2I(g)����H =-196.6 kJ��mol-1����һ���ݻ�����������У�����2 mol H��1 mol O2��ʹ֮��ַ�Ӧ���ų�������Ϊ��H1����H1�� ��H(�����������������)�������ǣ�__________________________��
2I(g)����H =-196.6 kJ��mol-1����һ���ݻ�����������У�����2 mol H��1 mol O2��ʹ֮��ַ�Ӧ���ų�������Ϊ��H1����H1�� ��H(�����������������)�������ǣ�__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012�갲���ߺ�һ�и߶���ѧ�����п����Ŀƻ�ѧ�Ծ����������� ���ͣ������
��5�֣���������ѧ��ѧ�г������ʵ�ת����ϵͼ�У���֪������AΪ����ɫ���塢BΪ�������ʣ�D��E��M�dz������嵥�ʣ�����MΪ����ɫ��N��θ����Ҫ�ɷ֣���ҵ����E��M����ȡN�����ƶϣ�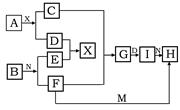
��1��д�����л�ѧʽ A I
��2��Gת��ΪI������Ϊ
��3��F+M��H���ӷ���ʽΪ
��4��A+X��C+D�Ļ�ѧ����ʽΪ________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���½���³ľ�����������һ������Բ��黯ѧ�Ծ��������棩 ���ͣ��ƶ���
��֪������A�ǻ���ɫ���壬C�ǵ���ɫ���壬EΪ���ɫ���塣�ں��ʵķ�Ӧ�� ���£����ǿ�����ͼ���з�Ӧ��
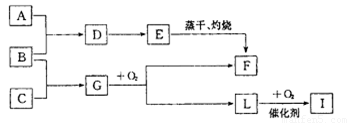
(1) ��ͼF���ʵĻ�ѧʽ________
(2) ��Ҫд����D�Ʊ�E�����ʵ���������_______��
(3) д��B+ C��G��һ�������·����Ļ�ѧ��Ӧ����ʽ_______
(4) д��B�ڳ�ʪ�Ŀ����з����绯ѧ��ʴʱ�������ĵ缫��Ӧʽ��_______
(5) ��һ���¶��£�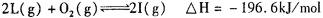 ����һ���ݻ������ �����У�����2mol L��1molO2,ʹ֮��ַ�Ӧ���ų�������ΪQ����Q_______196. 6kJ (� >������������=������
����һ���ݻ������ �����У�����2mol L��1molO2,ʹ֮��ַ�Ӧ���ų�������ΪQ����Q_______196. 6kJ (� >������������=������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com