
(9��)(1)1 mol��̬�����Ӻ�1mol��̬�����ӽ������1 mol�Ȼ��ƾ����ͷų�������Ϊ�Ȼ��ƾ���ľ����ܡ�
�������Ȼ�ѧ����ʽ�У���ֱ�ӱ�ʾ���Ȼ��ƾ��徧���ܵ���____________��
A��Na��(g)��Cl��(g)�D��NaCl(s)����Q
B��Na(s)��Cl2(g)�D��NaCl(s)����Q1
C��Na(s)�D��Na(g)����Q2
D��Na(g)��e���D��Na��(g)����Q3
E.Cl2(g)�D��Cl(g)����Q4
F��Cl(g)��e���D��Cl��(g)����Q5
��д����Q1���Q����Q2����Q3����Q4����Q5֮��Ĺ�ϵʽ________________________________________________________________________
(2)���淴Ӧ��aA(g)��bB(g) ??cC(g)��dD(g)��ȡa mol A��b mol B����V L�ܱ������У�2min���������A��Ũ��Ϊx mol��L��1��������C��Ũ�ȱ仯����ʾ���ʱ���ڷ�Ӧ��ƽ������ӦΪ__________ __��
 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(9��)��x��Y��Z��M��R��Q���ֶ���������Ԫ�أ�������Ϣ���±���ʾ��
��ش��������⣺
(1)R��Ԫ�����ڱ��е�λ����________________________________.
(2)���ݱ��������Ʋ⣬Y��ԭ�Ӱ뾶����С��Χ��_________________________.
(3)Z��M��Q�ļ����ӵ����Ӱ뾶�Ĵ�С˳��Ϊ________________ (��Ԫ�ط��ű�ʾ)��
(4)Y��R��ȣ��ǽ����Խ�ǿ����_________ (��Ԫ�ط��ű�ʾ)��������ʵ��֤����һ��
�۵���__________ (ѡ����ĸ���)��
a��������Y�ĵ��ʳʹ�̬��R�ĵ��ʳ���̬
b���ȶ���XR>YX��
c��Y��R�γɵĻ�������Y������
(5)X��M��Z����Ԫ����ɵĻ������к��еĻ�ѧ��Ϊ________��д��R�ĵ�������
���������ˮ��Һ��Ӧ�����ӷ���ʽ��
__________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
һ�ֻ�϶����������Էֱ��õ綯������ȼ�����߶��߽���ƶ����֡��������»����ʱ���綯���ṩ�ƶ������������͵����ģ���ɲ��������ʱ��ȼ���ṩ�ƶ�����ʹ�綯�����ڳ��״̬��Ŀǰ��ȼ��������Ϊȼ�ϣ��綯��һ��ʹ�������أ�KOH�����Һ����
�Է����ش��������⣺
��1����֪������ɲ��������ʱ�����������缫��Ӧ�ֱ�Ϊ��
�缫��M+H2O+e-��MH+OH-��MΪ����Ͻ�MHΪ��������ԭ�ӵĴ���Ͻ�
�ҵ缫��Ni��OH��2+OH--e����NiOOH+H2O
������һ�����мס������缫�����Ʒֱ��ǣ��ף� ���ң� ��
��2�����������»����ʱ�����������缫��Ӧ�ֱ�Ϊ��
�缫�� ���ҵ缫�� ��
�缫��Χ��Һ��pH�仯�ǣ�ѡ������䡱��С"����ͬ���� ���� ��
��3����ȼ������ʱ��Ϊ�������Ͳ���ȫȼ�ջ������Ⱦ������CO����֪�ڳ��³�ѹ�£�
C8 H18��1��+25��2 O2��g��=8CO2��g��+9 H2O��g������H=-5121.9kJ��mol
2CO��g��+O2��g��=2CO2��g������H=-566.0kJ��mol
H2 O��g��=H2O��1������H=-44.0kJ��mol
д�����Ͳ���ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ�� ��
��4��Ϊ��������β���е�һ����̼��Ũ�ȣ��ɲ�ȡ��������������������һ����ȼ����ͨ�����з�Ӧ��ʵ��ת����
2 CO��g��+O2��g��= 2CO2��g��
��֪���¶�ΪT�������£�����ȼ���л�ѧ��Ӧ���ʣ�����=v���棩ʱ��������Ũ�ȴ������к㶨��ϵ��
���¶�ΪT�������£���ij�������˲�ȼ����CO��CO2��Ũ�ȷֱ�Ϊ1.0��10-5mol��L-1��1.01��10-4mol��L-1��Ҫ�ڸ��¶���ʹ����β����CO��Ũ�Ƚ�Ϊ1.0��10-6mol��L-1����ȼ����Ӧ���ϲ���O2����ʹO2Ũ�ȱ����� mol��L-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ӱ�ʡ��ɽһ�и߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
(9��)
(1)д���������ʵĽṹ��ʽ��2,3,3���������� ��2��4-���ȼױ��Ľṹ��ʽΪ ����-2-��ϩ�Ľṹ��ʽΪ ��
(2)ij��״�л����к���n����CH2����m��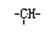 ��a ����CH3������Ϊ��OH����OH �ĸ���Ϊ
��a ����CH3������Ϊ��OH����OH �ĸ���Ϊ
(3)��������4��̼ԭ�ӵ�ij����������2��ͬ���칹�壬������̼ͬԭ������������Ҳ��4��̼ԭ�ӵĵ�ϩ����ͬ���칹���� ��
(4)����Ȳ����ȩ��ɵĻ�����壬���ⶨ���к�̼Ԫ�ص���������Ϊ72%��������������Ԫ�ص���������Ϊ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�������ѧ�ڻ�ѧһ�ָ�ϰ�����Ϳɳ�����չ��ר���ۺϲ��ԣ��ս̰棩 ���ͣ�ʵ����
(9��)A��B��C����ѧ���������ַǽ������ʣ�������BΪ��ɫ���壬A��CΪ���壬�ס��ҡ���Ϊ��ѧ���������ֻ����������C���������У�A��B��C��ס��ҡ���֮���ת����ϵ����ͼ��ʾ��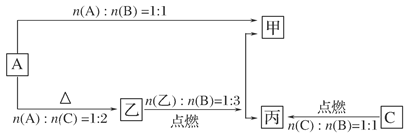
(1)д��Cת��Ϊ���Ļ�ѧ����ʽ________________________________________��
(2)�����£�����ͼװ��װ�ã�����������ѹǿ����ʱ��ˮ����Һ����M����ȡ����Ƥ�����ڲ���ȼ�ճ��м����ң�����B�г��ȼ�գ�ˮ����������ܡ�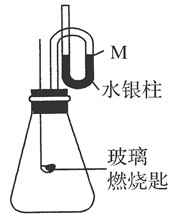
��Ҫ�ش��������⣺
��д����ȼ�յĻ�ѧ����ʽ��_____________________________________________��
��ˮ����������ܵ�ԭ����______________________________________________��
�ۻ���Ϩ�����һ��ʱ��ָ���ԭ�¶ȣ��ҹ�ˮ����λ��Ӧ����________��
A������M��������B������M��������C������M��
����ѡ���Ҫ˵������_______________________________________________��
������ͼ��ʾװ�ü�����ȼ�յIJ���A��B��C��D����ʢ�ŵ�ҩƷ�����ǣ�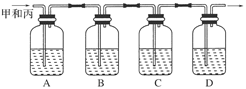
A��________��B��________��C��________��D��________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�������ѧ�ڻ�ѧһ�ָ�ϰ�����Ϳɳ�����չ��ר���ۺϲ��ԣ��ս̰棩 ���ͣ�ʵ����
(9��)A��B��C����ѧ���������ַǽ������ʣ�������BΪ��ɫ���壬A��CΪ���壬�ס��ҡ���Ϊ��ѧ���������ֻ����������C���������У�A��B��C��ס��ҡ���֮���ת����ϵ����ͼ��ʾ��

(1)д��Cת��Ϊ���Ļ�ѧ����ʽ________________________________________��
(2)�����£�����ͼװ��װ�ã�����������ѹǿ����ʱ��ˮ����Һ����M����ȡ����Ƥ�����ڲ���ȼ�ճ��м����ң�����B�г��ȼ�գ�ˮ����������ܡ�

��Ҫ�ش��������⣺
��д����ȼ�յĻ�ѧ����ʽ��_____________________________________________��
��ˮ����������ܵ�ԭ����______________________________________________��
�ۻ���Ϩ�����һ��ʱ��ָ���ԭ�¶ȣ��ҹ�ˮ����λ��Ӧ����________��
A������M��������B������M��������C������M��
����ѡ���Ҫ˵������_______________________________________________��
������ͼ��ʾװ�ü�����ȼ�յIJ���A��B��C��D����ʢ�ŵ�ҩƷ�����ǣ�

A��________��B��________��C��________��D��________
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com