
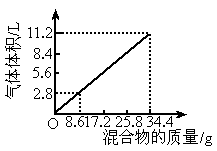
��100mL NaOH��Һ�м���NH4NO3��(NH4)2SO4�Ĺ���������ȳ�ַ�Ӧ����ͼ��ʾ����Ļ���������������������������״�����Ĺ�ϵ��
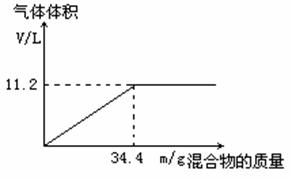 (1) NaOH��Һ�����ʵ���Ũ��Ϊ ��
(1) NaOH��Һ�����ʵ���Ũ��Ϊ ��
(2)��NaOH��Һ�����Ϊ80mL���������������Ϊ34.4g����ַ�Ӧ���������������ڱ�״����Ϊ ����
(3)��NaOH��Һ�����Ϊ120mL�����������������Ϊ34.4g����ַ�Ӧ���������������ڱ�״����Ϊ ����
 ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
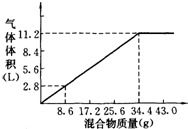 ��100mL NaOH��Һ�м���NH4NO3�ͣ�NH4��2SO4�Ĺ���������ȳ�ַ�Ӧ����ͼ��ʾ����Ļ��������������������������״�����Ĺ�ϵ���Լ��㣺
��100mL NaOH��Һ�м���NH4NO3�ͣ�NH4��2SO4�Ĺ���������ȳ�ַ�Ӧ����ͼ��ʾ����Ļ��������������������������״�����Ĺ�ϵ���Լ��㣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�038
��100mL NaOH��Һ�м���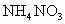 ��
��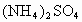 �Ĺ���������ȳ�ַ�Ӧ����ͼ��ʾ����Ļ�����������������������(��״��)�Ĺ�ϵ���Լ��㣺
�Ĺ���������ȳ�ַ�Ӧ����ͼ��ʾ����Ļ�����������������������(��״��)�Ĺ�ϵ���Լ��㣺

(1)NaOH�����ʵ���Ũ��Ϊ_______________________��
(2)��NaOH�ܵ����Ϊ140mL�����������������51.6gʱ����ַ�Ӧ��������������(��״��)Ϊ______________L��
(3)��NaOH��Һ�����Ϊ180mL���������������Ϊ51.6gʱ����ַ�Ӧ��������������(��״��)______________L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�058
��100mL NaOH��Һ�м���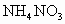 ��
�� �Ĺ���������ȳ�ַ�Ӧ����ͼ��ʾ����Ļ�����������������������(��״��)�Ĺ�ϵ���Լ��㣺
�Ĺ���������ȳ�ַ�Ӧ����ͼ��ʾ����Ļ�����������������������(��״��)�Ĺ�ϵ���Լ��㣺
(1)NaOH�����ʵ���Ũ��Ϊ_______________________��
(2)��NaOH�ܵ����Ϊ140mL�����������������51.6gʱ����ַ�Ӧ��������������(��״��)Ϊ______________L��
(3)��NaOH��Һ�����Ϊ180mL���������������Ϊ51.6gʱ����ַ�Ӧ��������������(��״��)______________L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014������и�һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�������
(6��)��100ml NaOH��Һ�м���NH4HCO3�ͣ�NH4��2SO4�Ĺ���������ȳ�ַ�Ӧ����ͼ��ʾ����Ļ���������Ͳ������������(��״��)�Ĺ�ϵ���Լ��㣺

(1)NaOH��Һ�����ʵ���Ũ��?
(2)��NaOH��Һ�����Ϊl40mL,��������������51.6gʱ����ַ�Ӧ��������������Ϊ��������
(3)��NaOH��Һ���Ϊ180mLʱ���������������Ϊ51.6gʱ����ַ�Ӧ��������������Ϊ������?
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com