��2009?��������ģ����ͼ��A��B��C�ֱ���ij����С����Ƶ���ȡ������������Ȫʵ�������װ��ʾ��ͼ����ȡNH
3ѡ���Լ���ͼ��ʾ���ش��������⣺

��1����Aͼ��ʾ��װ�ÿ��Ʊ������NH
3�ٷ�Ӧ�Ļ�ѧ����ʽΪ��
2NH
4Cl+Ca��OH��
2CaCl
2+2NH
3��+2H
2O
2NH
4Cl+Ca��OH��
2CaCl
2+2NH
3��+2H
2O
��װ�����ռ�NH
3���Թܿڷ������ŵ�������
��ֹ����������ʹNH3�����Թ�
��ֹ����������ʹNH3�����Թ�
��
�ڸ�����и�����ܷ������ˮCaCl
2����
����
��������
CaCl2+8NH3=CaCl2?8NH3
CaCl2+8NH3=CaCl2?8NH3
��
��2����Bͼ��ʾ��װ�ÿɿ�����ȡ�ϴ���NH
3������Ȫʵ�飮����Bͼ��ʾ��װ�ü��Լ��ش��������⣺
���û�ѧ����ʽ��ʾŨ��ˮ����CaO���д���NH
3�ݳ��Ĺ��̣�
CaO+H
2O=Ca��OH��
2����Ӧ���ȣ�NH
3?H
2O
NH
3��+H
2O
CaO+H
2O=Ca��OH��
2����Ӧ���ȣ�NH
3?H
2O
NH
3��+H
2O
�ڼ���NH
3�Ƿ��ռ�����ʵ�鷽���ǣ�
�ò�����պȡ����Ũ������ռ�NH3���Թܿڣ����������̣�˵���Թ����ռ���NH3����֮����û���ռ�����
����ʪ��ĺ�ɫʯ����ֽ�����ռ�NH3���Թܿڣ���ʪ��ĺ�ɫʯ����ֽ��������˵��NH3���ռ�������֮����û���ռ�����
�ò�����պȡ����Ũ������ռ�NH3���Թܿڣ����������̣�˵���Թ����ռ���NH3����֮����û���ռ�����
����ʪ��ĺ�ɫʯ����ֽ�����ռ�NH3���Թܿڣ���ʪ��ĺ�ɫʯ����ֽ��������˵��NH3���ռ�������֮����û���ռ�����
��
��3����Cͼ��ʾ��װ�ý�����Ȫʵ�飬�ϲ���ƿ�ѳ������ﰱ��������ˮ����IJ�����
��ֹˮ�м�����ͷ�ι��е�ˮ
��ֹˮ�м�����ͷ�ι��е�ˮ
����ʵ���ԭ���ǣ�
NH3�����ܽ���ˮ����ʹ��ƿ��ѹǿѸ�ټ�С
NH3�����ܽ���ˮ����ʹ��ƿ��ѹǿѸ�ټ�С
��
�����Cװ����ƿ��NH
3����������ͬ״������ͬ���H
2������10��������Ȫʵ����Ϻ���ƿ��ˮ����������ƿ�ݻ���
�������֮��������


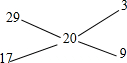 �����Կ����Ͱ��������֮��Ϊ1��3������Ȫʵ����Ϻ���ƿ��ˮ����������ƿ�ݻ���
�����Կ����Ͱ��������֮��Ϊ1��3������Ȫʵ����Ϻ���ƿ��ˮ����������ƿ�ݻ���


