
��ͬѧ��ͨ����ͼ��ʾװ��(�г�װ������ȥ)����ʵ�飬�о��ƶ�SO2��Na2O2��Ӧ�IJ��

�ش��������⣺
(1)װ��B������___________________________________________________��
װ��D������_____________________________________________________��
(2)��μ��鷴Ӧ���Ƿ���O2����______________________________________��
(3)����Na2O2�ѷ�Ӧ��ȫ��Ϊȷ��C�й������ijɷ֣����������ʵ�飺
 ��Ba(NO3)2��Һ ������ϡHNO3
��Ba(NO3)2��Һ ������ϡHNO3
�ó����ۣ�������Na2SO4.
�÷����Ƿ����____________(���ǻ��)��������_____________________-��
(4)�������������������һ����ȷ������ɷֵ�ʵ�鷽������д�±�(�ɲ�����)
��������(�����Լ�) | ʵ������ | ���� |
|
|
|
2 |
|
|
3 |
|
|
4 |
|
|
(1)B������SO2���壬��ֹ�϶��ˮ������Na2O2��Ӧ��D����ֹ�����е�ˮ�����Ͷ���
��̼����Cװ����Na2O2��Ӧ��ͬʱ���չ�����SO2��������Ⱦ������
(2)�ô��������ľ����������ܿ�a���۲����Ƿ�ȼ�ա�
(3)��HNO3�������ԣ��ݴ˲���ȷ��������NaSO3����Na2SO4���߶����С�
(4)
��������(�����Լ�) | ʵ������ |
|
1����C�еĹ��������Һ |
|
|
2�������������� | ()������������ (2)������������ | ��Na2SO3 ��Na2SO3 |
3���ټ���BaCL2��Һ | (1) �����ɰ�ɫ���� (2) ������������ | ��Na2SO4 ��Na2SO4 |


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ����������ѧ�߶�5���¿���ѧ�Ծ����������� ���ͣ�ʵ����
ͬѧ��ͨ����ͼ��ʾװ�ã��г�װ������ȥ������ʵ�飬�о��ƶ�SO2��Na2O2��Ӧ�IJ��
�ش��������⣺
��1��װ��B������___________________________________________________��
װ��D�������չ�����SO2���ʲô����___________________________��
��2����μ��鷴Ӧ���Ƿ���O2����______________________________________��
��3������Na2O2�ѷ�Ӧ��ȫ��Ϊȷ��C�й������ijɷ֣����������ʵ�飺
�ó����ۣ�������Na2SO4.
�÷����Ƿ����____________�����ǻ��������_____________________-��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��ӱ�ʡ������ѧ�ڶ������Ի�ѧ�Ծ��������棩 ���ͣ������
��.±�����±�����ڹ�ҵ������������Ҫ�����á�ijС��Ϊ̽������һЩ�ε���
�ʣ��������ϲ�����ʵ�顣�����������£�
�� BrO3�� + 6I�� + 6H+ = 3I2 + Br��+ 3H2O �� 2BrO3�� + I2 = 2IO3�� + Br2
�� IO3�� + 5I�� + 6H+ = 3I2 + 3H2O �� 2IO3�� + 10Br��+ 12H+ = I2 + 5Br2 + 6H2O
ʵ�����£�
|
���� |
���� |
|
��.��ʢ��30 mL 0.2 mol��L-1 KI��Һ����ƿ�����ε��뼸�ε�����Һ������ϡ���ᣬ���õζ�����μ���KBrO3��Һ |
����KBrO3��Һ���룬��Һ����ɫ��Ϊ��ɫ������,���ձ��ֲ��� |
|
��.������������Һ�е���KBrO3��Һ |
��Һ����ɫ����ȥ |
��ش�
��1�����������еķ�Ӧ��~�ܲ������ѧ֪ʶ���ж�IO3����BrO3����I2��Br2����������ǿ������˳���� ��KBrO3��Һ��KBr��Һ�����������·�Ӧ�����ӷ���ʽ�� ��
��2������y��ʾ��ƿ�к������ʵ����ʵ��� ����x��ʾ������KBrO3�����ʵ���������ͼ�л�����������ʵ�������y��x�ı仯���ߣ�Ҫ����ͼ�б���յ����꣩��

��.��̼�����к��������������ƣ��ס�����λͬѧ����ȡһ�������ĸ���Ʒ����������ͼ��ʾ�����ⶨ��Ʒ�Ĵ��ȡ�����������˳��
��ͬѧ���ݡ��ࡪ�ۡ��ߡ��ܣ� ��ͬѧ���ݡ��ۡ��ڡ�

��֪����̼���ƣ�Na2CO4�����������Ʒֱ������ϡ���ᷴӦ�Ļ�ѧ����ʽ���£�
2Na2CO4��2H2SO4=2Na2SO4��2CO2����O2����2H2O;
2Na2O2��2H2SO4=2Na2SO4��O2����2H2O��
��1����ͬѧ��ͨ��ʵ���õ�������____________����ѡ�õ�װ��________������ţ���û�б�Ҫ�ġ�
��2����ͬѧ��ͨ��ʵ���õ�������________________��������Ϊ������õ����ݼ������ʵ��������ƫ�ߣ�ԭ����________________�� Ϊ�˲��ȷ��ʵ�����ݣ����㽫��ͬѧ��ʵ����ƽ��иĽ���д������ѡ������������˳��ÿ���������ʹ��һ�Σ�Ҳ���Բ��ã���________________������ţ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ�߶�5���¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
ͬѧ��ͨ����ͼ��ʾװ�ã��г�װ������ȥ������ʵ�飬�о��ƶ�SO2��Na2O2��Ӧ�IJ��

�ش��������⣺
��1��װ��B������___________________________________________________��
װ��D�������չ�����SO2���ʲô����___________________________��
��2����μ��鷴Ӧ���Ƿ���O2����______________________________________��
��3������Na2O2�ѷ�Ӧ��ȫ��Ϊȷ��C�й������ijɷ֣����������ʵ�飺

�ó����ۣ�������Na2SO4.
�÷����Ƿ����____________�����ǻ��������_____________________-��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͬѧ��ͨ����ͼ��ʾװ�ã��г�װ������ȥ������ʵ�飬�о��ƶ�![]() ��
��![]() ��Ӧ�IJ��
��Ӧ�IJ��
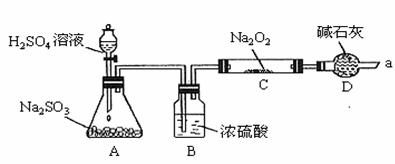
�ش��������⣺
��1��װ��B������____________________________________________________��
װ��D������____________________________________________________��
��2����μ��鷴Ӧ���Ƿ���![]() ���� ��
���� ��
��3������![]() �ѷ�Ӧ��ȫ��Ϊȷ��C�й������ijɷ֣����������ʵ�飺
�ѷ�Ӧ��ȫ��Ϊȷ��C�й������ijɷ֣����������ʵ�飺
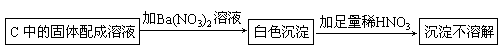
�ó����ۣ�������![]() ��
��
�÷����Ƿ����_____�����ǻ��������_______________________________��
��4�������������������������һ����ȷ������ɷֵ�ʵ�鷽������д�±����ɲ���������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com