
ÔÚ10.0ˇćşÍ2´105PaµÄĚőĽţĎÂŁ¬·´Ó¦aA(g)ƒdD(g)+eE(g)˝¨Á˘Ć˝şâşóŁ¬ÔÚ˛»ĽÓČëČÎşÎÎďÖʵÄĚőĽţĎÂÖđ˛˝Ôö´óĚĺϵµÄѹǿŁ¨Î¶ČάłÖ˛»±äŁ©ˇŁ±íÖĐÁĐłö˛»Í¬ŃąÇżĎ·´Ó¦˝¨Á˘Ć˝şâʱÎďÖĘDµÄŨ¶ČŁş
ѹǿ/Paˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇ 2´105ˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇ 5´105ˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇ 1´106
DµÄŨ¶Č/molˇÁL-1ˇˇˇˇˇˇˇˇ 0.085ˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇ 0.20ˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇˇ 0.44
¸ůľÝ±íÖĐĘýľÝŁ¬»Ř´đĎÂÁĐÎĘĚ⣺
Ł¨1Ł©ŃąÇż´Ó2´105PaÔöĽÓµ˝5´105PaʱŁ¬Ć˝şâĎň_______·´Ó¦·˝ĎňŇƶŻŁ¨ĚŐýˇ±»ňˇ°Ä桱Ł©Ł¬ŔíÓÉĘÇ_______ˇŁ
Ł¨2Ł©ŃąÇż´Ó5´105PaÔöĽÓµ˝1´106PaʱŁ¬Ć˝şâĎň_______·´Ó¦·˝ĎňŇƶŻŁ¨ĚŐýˇ±»ňˇ°Ä桱Ł©Ł¬ŔíÓÉĘÇ_______ˇŁ

| Ä꼶 | ¸ßÖĐżÎłĚ | Ä꼶 | łőÖĐżÎłĚ |
| ¸ßŇ» | ¸ßŇ»Ăâ·ŃżÎłĚÍĆĽöŁˇ | łőŇ» | łőŇ»Ăâ·ŃżÎłĚÍĆĽöŁˇ |
| ¸ß¶ţ | ¸ß¶ţĂâ·ŃżÎłĚÍĆĽöŁˇ | łő¶ţ | łő¶ţĂâ·ŃżÎłĚÍĆĽöŁˇ |
| ¸ßČý | ¸ßČýĂâ·ŃżÎłĚÍĆĽöŁˇ | łőČý | łőČýĂâ·ŃżÎłĚÍĆĽöŁˇ |
żĆÄżŁş¸ßÖĐ»ŻŃ§ Ŕ´Ô´Łş ĚâĐÍŁşÔĶÁŔí˝â
 CH3OHŁ¨gŁ©ˇ÷H1=-116kJ?molˇĄ1
CH3OHŁ¨gŁ©ˇ÷H1=-116kJ?molˇĄ1| 1 |
| 2 |
| 1 |
| 2 |

| ʵŃé±ŕşĹ | TŁ¨ˇćŁ© | nŁ¨COŁ©/nŁ¨H2Ł© | PŁ¨MPaŁ© |
| 1 | 150 | 1/3 | 0.1 |
| 2 | a | 1/3 | 5 |
| 3 | 350 | b | 5 |
 »ŻµÄÇúĎßÍĽŁ¬ÇëÖ¸Ă÷ÍĽÖеÄѹǿP1=
»ŻµÄÇúĎßÍĽŁ¬ÇëÖ¸Ă÷ÍĽÖеÄѹǿP1= COŁ¨gŁ©+2H2Ł¨gŁ©·´Ó¦µÄĆ˝şâłŁĘýÎŞ
COŁ¨gŁ©+2H2Ł¨gŁ©·´Ó¦µÄĆ˝şâłŁĘýÎŞ| 4a2 |
| V2 |
| 4a2 |
| V2 |
˛éż´´đ°¸şÍ˝âÎö>>
żĆÄżŁş¸ßÖĐ»ŻŃ§ Ŕ´Ô´Łş ĚâĐÍŁşÔĶÁŔí˝â
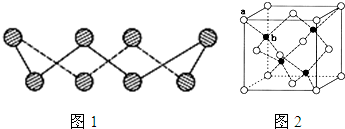
| SO | 2- 3 |
| ||
| (540.0ˇÁ10-10cm)3 |
| ||
| (540.0ˇÁ10-10cm)3 |
| 270.0 | ||
|
135.0ˇÁ
| ||
sin
|
| 3 |
| 270.0 | ||
|
135.0ˇÁ
| ||
sin
|
| 3 |
˛éż´´đ°¸şÍ˝âÎö>>
żĆÄżŁş¸ßÖĐ»ŻŃ§ Ŕ´Ô´Łş2013-2014ѧÄę°˛»Őʡˇ°˝»´Ę®ĐŁˇ±Đ×÷Ěĺ¸ßČýÉĎѧĆÚµÚŇ»´ÎÁŞżĽ»ŻŃ§ĘÔľíŁ¨˝âÎö°ćŁ© ĚâĐÍŁşĚîżŐĚâ
ŇŃÖŞŁş˘Ů±ę׼״żöĎÂŁ¬1Ěĺ»ýË®ÖĐ×î¶ŕÄÜČÜ˝â500Ěĺ»ýµÄHClŁ»
˘Ú±ĄşÍNaClČÜŇşµÄŨ¶ČÔĽÎŞ5.00 molˇ¤LŁ1ˇŁ
ÔÚ±ę׼״żöĎÂŁ¬˝«448 L HClĆřĚĺČÜÓÚ1 LË®ÖĐŁ¬ËůµĂČÜŇşAµÄĂܶČÎŞ1.20 gˇ¤cm-3Ł¬ÔňČÜŇşAÖĐHClµÄÎďÖʵÄÁżĹ¨¶ČÎŞ ˇŁ(±ľĚâĽĆËă˝áąűľůȡČýλÓĐЧĘý×Ö)
Ł¨1Ł©ČôĘąClŁĹ¨¶ČÓëČÜŇşAÖеÄClŁĹ¨¶ČĎŕµČŁ¬ÔňÔÚ1 L NaCl±ĄşÍČÜŇşÖĐ»ąÓ¦ČÜ˝âÔĽ L±ę׼״żöĎÂHClĆřĚĺ (ČÜŇşĚĺ»ý±ä»ŻşöÂÔ˛»ĽĆ)ˇŁ
Ł¨2Ł©Čˇ10.0 mLČÜŇşAϡĘÍłÉ500 mLČÜŇşBŁ¬ÔňČÜŇşBÖĐHClµÄÎďÖʵÄÁżĹ¨¶ČÎŞ ˇŁ
Ł¨3Ł©ÔÚČÜŇşBµÄĹäÖĆąýłĚÖĐŁ¬ĘąÓĂÇ°±ŘĐëĽě˛éĘÇ·ń©ҺµÄŇÇĆ÷ÓĐ Ł»ĎÂÁĐĹäÖƲŮ×÷Ł¬ÔěłÉČÜŇşBŨ¶ČĆ«µÍµÄĘÇ_______________(ѡĚîĐňşĹ)ˇŁ
a.ČÝÁżĆżÓĂŐôÁóˮϴµÓşóδ¸ÉÔď
b.ÁżČˇČÜŇşAµÄÁżÍ˛ÓĂŐôÁóˮϴµÓşóδ¸ÉÔď
c.¶¨ČÝʱŁ¬¸©ĘÓŇşĂćĽÓË®ÖÁżĚ¶ČĎß
d.ĽÓË®¶¨ČÝʱҺĂ治É÷ł¬ąýżĚ¶ČĎߣ¬Á˘Ľ´ÓĂ˝şÍ·µÎąÜÎüłö˛ż·ÖˮʹҺĂć¸ŐşĂ´ďżĚ¶ČĎß
e.ÉŐ±ÖĐČÜŇşŇĆČëČÝÁżĆżşóŁ¬Î´ÓĂˮϴµÓÉŐ±şÍ˛ŁÁ§°ôĽ´¶¨ČÝ
˛éż´´đ°¸şÍ˝âÎö>>
żĆÄżŁş¸ßÖĐ»ŻŃ§ Ŕ´Ô´Łş¸ßżĽŐćĚâ ĚâĐÍŁşĚîżŐĚâ
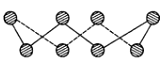
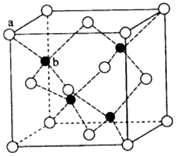
˛éż´´đ°¸şÍ˝âÎö>>
żĆÄżŁş¸ßÖĐ»ŻŃ§ Ŕ´Ô´Łş ĚâĐÍŁş
(15·Ö)
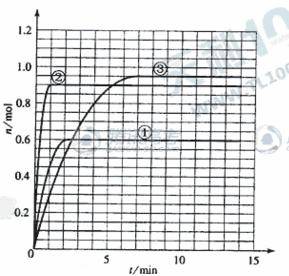
ijζČʱŁ¬ÔÚ2LĂܱŐČÝĆ÷ÖĐĆř̬ÎďÖĘXşÍY·´Ó¦ÉúłÉĆř̬ÎďÖĘZŁ¬ËüĂǵÄÎďÖʵÄÁżËćʱĽäµÄ±ä»ŻČç±íËůĘľˇŁ
Ł¨1Ł©¸ůľÝϱíÖĐĘýľÝŁ¬ÔÚ´đĚ⿨¸ĂĚâĎŕӦλÖĂÉĎ»łöXˇ˘Yˇ˘ZµÄÎďÖʵÄÁżŁ¨nŁ©ËćʱĽäŁ¨tŁ©±ä»ŻµÄÇúĎߣş
| t/min | X/mol | Y/mol | Z/mol |
| 0 | 1.00 | 1.00 | 0.00 |
| 1 | 0.90 | 0.80 | 0.20 |
| 3 | 0.75 | 0.50 | 0.50 |
| 5 | 0.65 | 0.30 | 0.70 |
| 9 | 0.55 | 0.10 | 0.90 |
| 10 | 0.55 | 0.10 | 0.90 |
| 14 | 0.55 | 0.10 | 0.90 |

(2)ĚĺϵÖĐ·˘Éú·´Ó¦µÄ»ŻŃ§·˝łĚĘ˝ĘÇ___________________________;
(3)ÁĐĘ˝ĽĆËă¸Ă·´Ó¦ÔÚ0-3minʱĽäÄÚ˛úÎďZµÄĆ˝ľů·´Ó¦ËŮÂĘŁş_______________;
(4)¸Ă·´Ó¦´ďµ˝Ć˝şâʱ·´Ó¦ÎďXµÄת»ŻÂĘaµČÓÚ___________________________;
(5)Čçąű¸Ă·´Ó¦ĘÇ·ĹČČ·´Ó¦ˇŁ¸Ä±äʵŃéĚőĽţŁ¨Î¶ȡ˘ŃąÇżˇ˘´ß»ŻĽÁŁ©µĂµ˝ZËćʱĽä±ä»ŻµÄÇúĎß1ˇ˘2ˇ˘3Ł¨ČçÓŇÍĽËůĘľŁ©ÔňÇúĎß1ˇ˘2ˇ˘3Ëů¶ÔÓ¦µÄʵŃéĚőĽţ¸Ä±ä·Ö±đĘÇŁş
˘Ů_________________ ˘Ú_______________ ˘Ű__________________
˛éż´´đ°¸şÍ˝âÎö>>
ąúĽĘѧУÓĹѡ - Á·Ď°˛áÁбí - ĘÔĚâÁбí
şţ±±Ęˇ»ĄÁŞÍřÎĄ·¨şÍ˛»ÁĽĐĹϢľŮ±¨Ć˝Ě¨ | ÍřÉĎÓĐş¦ĐĹϢľŮ±¨×¨Çř | µçĐĹթƾٱ¨×¨Çř | ÉćŔúĘ·ĐéÎŢÖ÷ŇĺÓĐş¦ĐĹϢľŮ±¨×¨Çř | ÉćĆóÇÖȨľŮ±¨×¨Çř
ÎĄ·¨şÍ˛»ÁĽĐĹϢľŮ±¨µç»°Łş027-86699610 ľŮ±¨ÓĘĎ䣺58377363@163.com