
��10�֣����ϵ�֣�ݡ�����������������ʹ�����������÷�����з�ʽ�������µ�����ȼ�ϡ��������Ҵ����͡��Ҵ��������ƾ������������ס�С�������Ϊԭ�Ͼ����͡�������Ƴɵġ��Ҵ���һ����ˮ���ټ����������ͺ��γɱ���ȼ���Ҵ����������Ҵ����;��ǰѱ���ȼ���Ҵ������Ͱ�һ�����������γɵij���ȼ�ϡ�����й�֪ʶ������������⣺
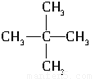
(1)�Ҵ��Ľṹ��ʽΪ_________________����������ʯ�ͷ������õĵͷе�������������е�̼ԭ����һ����C5��C11��Χ�ڣ������飬�����ʽΪ_________________����ͬ���칹��ṹ��ʽ�ֱ�Ϊ_______________________��________________________��_______________________��
(2)�Ҵ����ɺ����ۡ�(C6H10O5)n����ũ��Ʒ�������ס�С������ȣ������͡�������ã��������ơ������Ҫ���������ٵ���+ˮ ������(C6H12O6)
��������
������(C6H12O6)
�������� �Ҵ���
�Ҵ���
��д���������������ǵĻ�ѧ����ʽ��___________________________________��
(3)�Ҵ����ȼ�յIJ���Ϊ_____________��_____________��
(4)�����Ҵ����ͳ�Ϊ����ȼ�ϣ���ԭ����________________________________��
��1��1��5=5�� C2H5OH C5H12 CH3CH2CH2CH2CH3
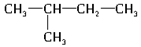
��2��(C6H10O5)n�����ۣ�+nH2O nC6H12O6�������ǣ�
��2�֣�
nC6H12O6�������ǣ�
��2�֣�
��3��CO2 H2O��2�֣�
��4������Ч��������β�����������ش�����Ⱦ�����ƻ��� ��1�֣�
����������1���Ҵ��к����ǻ������Խṹ��ʽΪC2H5OH������������ͨʽCnH2n��2��֪������ķ���ʽ��C5H12������������ͬ���칹�壬�ֱ���CH3CH2CH2CH2CH3��CH3CH2CH(CH3)2��C(CH3)4��
��2������ˮ�⼴���������ǣ�����ʽΪ(C6H10O5)n+nH2O nC6H12O6
nC6H12O6
�����ۣ� �������ǣ�
��3�������Ҵ������Ԫ�ؿ�֪���Ҵ���ȫȼ�յ���������CO2��ˮ��
��4�������Ҵ���ȼ�ղ����֪���Ҵ�����֮���Գ�Ϊ����ȼ�ϣ���ԭ������������Ч��������β�����������ش�����Ⱦ�����ƻ�����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� |
| ���� |
| ||
| Ҷ���� |
| ||
| Ҷ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)�Ҵ��Ľṹ��ʽΪ_____________����������ʯ�ͷ������õĵͷе�������������е�̼ԭ����һ����C5��C11��Χ�ڣ������飬�����ʽΪ__________________���ṹ��ʽ����ͬ���칹��ֱ�Ϊ_____________��_____________��_____________��
(2)�Ҵ����ɺ����ۡ�(C6H10O5)n����ũ��Ʒ�������ס�С������Ⱦ����͡�������á���д���ɵ������Ҵ��Ļ�ѧ����ʽ��
�ٵ���+ˮ![]() ������(C6H12O6)
������(C6H12O6)
��������![]() �Ҵ�
�Ҵ�
(3)���ۿ�����ɫֲ�ᆳ������õ�һϵ�����ﻯѧ��Ӧ�õ�����ˮ�Ͷ�����̼������������������ǣ��������������ɵ��ۡ����й�����õij�����_____________����������������������ǵĻ�ѧ����ʽ��__________________________________________________________��
(4)�Ҵ����ȼ�յIJ���Ϊ__________________��__________________��
(5)�����Ҵ����ͳ�Ϊ����ȼ�ϣ���ԭ����___________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)�Ҵ��Ľṹ��ʽΪ_______________����������ʯ�ͷ������õĵͷе�������������е�̼ԭ����һ����C5��C11��Χ�ڣ������飬�����ʽΪ_______________���ṹ��ʽ����ͬ���칹��ֱ�Ϊ_______________��_______________��_______________��
(2)�Ҵ����ɺ����ۡ�(C6H10O5)n����ũ��Ʒ�����ס�С������Ⱦ����͡�������á���д���ɵ������Ҵ��Ļ�ѧ����ʽ��
�ٵ���+ˮ![]() ������(C6H12O6)
������(C6H12O6)
��������![]() �Ҵ�
�Ҵ�
(3)���ۿ�����ɫֲ�ᆳ������õ�һϵ�����ﻯѧ��Ӧ�õ�����ˮ�Ͷ�����̼������������������ǣ��������������ɵ��ۡ����й�����õij�����_________________����������������������ǵĻ�ѧ����ʽ��_____________________________________________________��
(4)�Ҵ����ȼ�յIJ���Ϊ________________��________________��
(5)�����Ҵ����ͳ�Ϊ����ȼ�ϣ���ԭ����_________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꺣��ʡ������ѧ��һ��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
��10�֣����ϵ�֣�ݡ�����������������ʹ�����������÷�����з�ʽ�������µ�����ȼ�ϡ��������Ҵ����͡��Ҵ��������ƾ������������ס�С�������Ϊԭ�Ͼ����͡�������Ƴɵġ��Ҵ���һ����ˮ���ټ����������ͺ��γɱ���ȼ���Ҵ����������Ҵ����;��ǰѱ���ȼ���Ҵ������Ͱ�һ�����������γɵij���ȼ�ϡ�����й�֪ʶ������������⣺
(1)�Ҵ��Ľṹ��ʽΪ_________________����������ʯ�ͷ������õĵͷе�������������е�̼ԭ����һ����C5��C11��Χ�ڣ������飬�����ʽΪ_________________����ͬ���칹��ṹ��ʽ�ֱ�Ϊ_______________________��________________________��_______________________��
(2)�Ҵ����ɺ����ۡ�(C6H10O5)n����ũ��Ʒ�������ס�С������ȣ������͡�������ã��������ơ������Ҫ���������ٵ���+ˮ ������(C6H12O6) ��������
������(C6H12O6) �������� �Ҵ���
�Ҵ���
��д���������������ǵĻ�ѧ����ʽ��___________________________________��
(3)�Ҵ����ȼ�յIJ���Ϊ_____________��_____________��
(4)�����Ҵ����ͳ�Ϊ����ȼ�ϣ���ԭ����________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com