
(9��)��ͼ��ijͬѧ��Ƶķ��ȷ�Ӧ�Ĺ۲�װ�á�
��ʵ��˳���ǣ��ٰ�ͼ��ʾ��ʵ��װ�����Ӻá�
����U�ι��ڼ���������īˮ(��Ʒ��)��Һ����T�� �����У�ʹU�ι������ߵ�Һ�洦��ͬһˮƽ�棬�ټн������С������м���Թ���ʢ1 g�����ƣ�������2 mL���ҵ�����ˮ��ͬʱ�������м��ɹ۲졣
�Իش�(1)ʵ���й۲쵽��������
(2)��ʵ���б�����е�һ��ʵ�������
(3)��ʵ���ԭ����
________________________________________________________________________
(4)ʵ���з�Ӧ�Ļ�ѧ��Ӧ����ʽ��
(5)˵��CaO��H2O��������Ca(OH)2������֮��Ĺ�ϵ
(6)����ʵ����CaO����NaCl��ʵ�黹�ܷ�۲쵽��ͬ����____��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
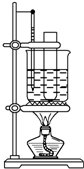 ʵ�����Ʊ��������ķ����DZ���Ũ�����Ũ����Ļ��Һ���ȵ�55�桫60�淴Ӧ����֪�����������Ļ��������������±���ʾ��
ʵ�����Ʊ��������ķ����DZ���Ũ�����Ũ����Ļ��Һ���ȵ�55�桫60�淴Ӧ����֪�����������Ļ��������������±���ʾ��| �۵� | �е� | ״̬ | |
| �� | 5.51�� | 80.1�� | Һ�� |
| ������ | 5.7�� | 210.9�� | Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
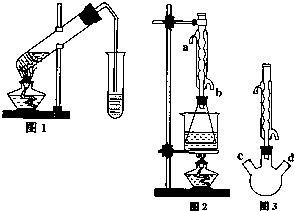 ������������Ҫ�Ĺ�ҵԭ�Ϻ��ܼ���ͨ�����������Ҵ������ᣮijѧϰС������ͼ1װ����ȡ���������ֲ�Ʒ���ٷ������������ĺ�����
������������Ҫ�Ĺ�ҵԭ�Ϻ��ܼ���ͨ�����������Ҵ������ᣮijѧϰС������ͼ1װ����ȡ���������ֲ�Ʒ���ٷ������������ĺ�����| �������� | �Ҵ� | ���� | |
| �е� | 77.1�� | 78.5�� | 117.9�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com