�����г�ʹ�õ����Ͻ��е��������ڵ�����������������ڹ�ҵ����������Ҫ���䴿�Ȳ��õ���98.2%������Ȼ�������е�����������Ϊ50%��70%��������ҪΪSiO
2��Fe
2O
3��CaO��MgO��Na
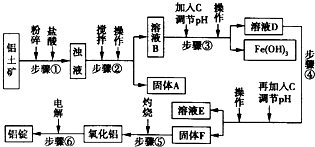
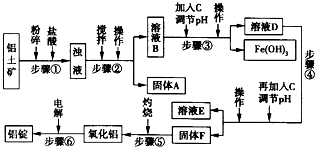
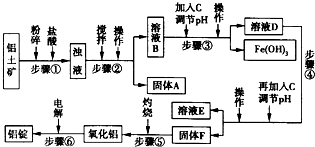
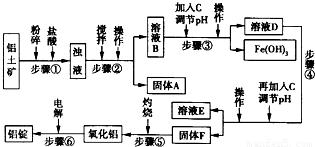
2O�ȣ���ҵ���������Ĺ�������ʾ��ͼ������ʾ��
һЩ�����↑ʼ��������ȫ������pH�����ʾ��
| ������ | Al��OH��3 | Fe��OH��3 | Mg��OH��2 |
| ��ʼ����pH�����ӳ�ʼŨ��Ϊ0.01mol��L�� | 4 | 2.3 | 10.4 |
| ��ȫ����pH������Ũ�ȣ�10-5mol/L�� | 5.2 | 4.1 | 12.4 |
��ش��������⣺
��1����������ʱ�������������ᷢ����Ӧ�����ӷ���ʽΪ______��
��2������ڢۢ��в���������Ϊ______��
��3������A�Ļ�ѧʽΪ______������C�Ļ�ѧʽΪ______����Һ�е�Na
+��Ca
2+��Mg
2+���ڲ���______�г�ȥ�ģ�
��4��������е�����ҺpH����ֵ��ΧΪ______��������е�����ҺpH����ֵ��ΧΪ______��
��5��������з�����Ӧ�Ļ�ѧ����ʽΪ______����ʯ4Al+3O
2��


 4Al+3O2�����ʴ�Ϊ��2Al2O3
4Al+3O2�����ʴ�Ϊ��2Al2O3 4Al+3O2����
4Al+3O2����