
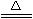
ͼ(��)

ͼ(��)
ͼ2-1
(1)NaHCO3���ȷֽ�Ļ�ѧ����ʽΪ����������������?
(2)����NaHCO3����ʱ���۲쵽�ձ��е������ǣ������ӷ���ʽ��?
(3)����ʵ��ʱ��X��Ӧ�����ҩƷΪ(��Na2CO3��NaHCO3)���������ǡ�?
(4)�����ͼ(2)װ�õ������ԣ��ɲ��á�
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
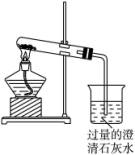
ͼ(��)
ͼ(��)
(1)NaHCO3���ȷֽ�Ļ�ѧ����ʽΪ______________________________��
(2)����NaHCO3����ʱ���۲쵽�ձ��е�������______________________________�������ӷ���ʽ______________________________��
(3)����ʵ��ʱ��X��Ӧ�����ҩƷΪ____________________(��Na2CO3��NaHCO3)����������__________________________________________________��
(4)�����ͼ(��)װ�õ������ԣ��ɲ���________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

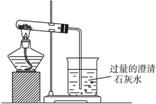
ͼ(1)

ͼ(2)
(1)NaHCO3���ȷֽ�Ļ�ѧ����ʽΪ_____________________________________________��
(2)����NaHCO3����ʱ���۲쵽�ձ��е�������__________________________�������ӷ���ʽ___________________________________��
(3)����ʵ��ʱ��X��Ӧ�����ҩƷΪ__________________ (��Na2CO3��NaHCO3)����������___________________________��
(4)�����ͼ(2)װ�õ������ԣ��ɲ���____________________________________��?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
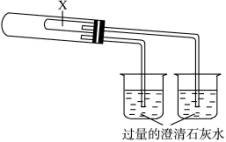
Ϊ��֤Na2CO3��NaHCO3�����ȶ��ԣ���ͳ����Ʒ������£���Na2CO3��NaHCO3�ֱ����ͼ(��)װ�õ��Թ��У�Ȼ����Ȳ�ͨ���۲�ʯ��ˮ�Ƿ�仯����֤Na2CO3��NaHCO3�����ȶ��ԣ�ij�о���ѧϰС���ʵ��װ���������¸Ľ���������ʵ�顱����װ����ͼ(��)��ʾ(����װ��δ����)����۲�ʵ��װ�ã�����ʵ��ԭ��������������⣺
ͼ(��)
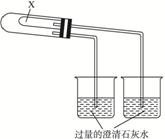
ͼ(��)
ͼ2-1
(1)NaHCO3���ȷֽ�Ļ�ѧ����ʽΪ����������������?
(2)����NaHCO3����ʱ���۲쵽�ձ��е������ǣ������ӷ���ʽ��?
(3)����ʵ��ʱ��X��Ӧ�����ҩƷΪ(��Na2CO3��NaHCO3)���������ǡ�?
(4)�����ͼ(2)װ�õ������ԣ��ɲ��á�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com