
�⻯��(CaH2)������һ�ִ�����ϣ��ǵ�ɽ�˶�Ա���õ���Դ�ṩ�������ڼ���ʱ���뵪����������Ӧ���⻯����ˮ������Ӧ�����������ƺ��������⻯��ͨ��������������Ƽ�����ȡ����ͼ��ģ����ȡװ�á�
��1�����й����⻯�Ƶ�������ȷ���� (ѡ�����)��
a���⻯���������ӵİ뾶С��Li+�İ뾶
b���⻯�Ƶ�ʽ��С���廯�⣬���ǰ�ߵ��۵�С�ں���
c���⻯��Ҳ�������ᷴӦ��������
d��������������ԭ����ֻ���л�ԭ��
��2����ͼAװ�����Ʊ��������õ�����Һ���ѡ�� (ѡ�����)��
a��ϡ���� b��ϡ���� c��ϡ���� d��������
��3��װ��D����ֱ���ܵ������� ��
��4��Ϊ��ȷ�Ͻ���װ��C�������Ѿ��������B��C֮���ٽ�һװ�ã���װ���м�����Լ��� ������Cװ��ǰҪ��H2�鴿���鴿�IJ����� ��
��5����ͬѧ��ΪֻҪװ�ú����������淶�Ϳ����ų����� (ѡ�����)��
a��Ca3N2 b��CaO c��Ca(OH)2
��6����ͬѧ����ͼװ�òⶨ�Ƶõ��⻯�ƵĴ��ȡ�����ȡ48g��Ʒ����������ˮ��Ӧ������ʱ��ע������������������Ϊ48.16 L(�ѻ���Ϊ��״��)�������ֻ�����������˷�Ӧ���������ͬѧ��ʵ�����ݼ����⻯�ƵĴ���(д���������) ��
��1��c
��2��b
��3��ƽ��ѹǿ����ֹ���浼�ܶ���
��4����ˮ����ͭ(������������)���ռ�һ�Թ����壬���ܿڿ����ƾ��ƻ��棬�����������ۡ�������
��5��a��b��c��
��6��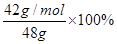 ��0.875
��0.875
���������������1��a�У��⻯����H-��Li+�ĵ��ӹ�����ͬ��Li�ĺ˵�����뾶С��a˵������ȷ��b��⻯�������Ӿ��壬�廯���Ƿ��Ӿ��壬���ǰ�ߵ��۵���ں��ߣ�����ȷ��d�������Ca��Ӧ������������d����ȷ��
��2���Ʊ�H2ʱ��ʹ��ϡ���ᣬ�����HCl���壬Ҳ����Ca��Ӧ��ʹ��ϡ���ᣬ����Ϊ������������H2�����Բ��С�
��3��ϴ��ƿ�в���Һ���ڲ��ĵ����������ͨ����ƽ��ѹǿ����ֹ���浼�ܶ���(��ֱ�������岻��)��
��4������ˮ������һ��ʹ����ˮ����ͭ(����ˮ����δ��ȥ�������ɫ)������ˮ���ռ�һ�Թ����壬���ܿڿ����ƾ��ƻ��棬�����������ۡ�������
��5����ʼͨ��H2�������ų�װ���еĿ�����û��N2��O2��ˮ�������������Ca3N2��CaO��Ca(OH)2�����ʡ�
��6��42g��mol-1 n(CaH2)+40 g��mol-1n(Ca)=48
2n(CaH2)+n(Ca)= 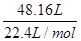 ��ã�n(CaH2)=1mol
��ã�n(CaH2)=1mol
���⻯�ƵĴ��ȣ�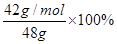 =87.5%��0.875��
=87.5%��0.875��
���㣺�����Ʊ�ʵ����ƺ����ۡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����ͼ��ʾ��װ����ȡ���ᴿ���ռ����е��������壬ͼ��a��b��c��ʾ��Ӧ����������Լ���δ����β�����������⣬���п��е��ǣ� ��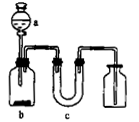
| | ���� | a | b | C |
| A | 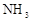 | Ũ��ˮ | ��ʯ�� | ��ʯ�� |
| B | 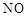 | ϡ���� | ͭƬ | ��ʯ�� |
| C | 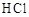 | Ũ���� | Ũ���� | �Ȼ��� |
| D | SO2 | Ũ���� | ͭƬ | �轺 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��ͼ����ȡ���ռ�ij�����ʵ��װ�ã���װ�ÿ�����
| A����Ũ������Ȼ��Ʒ�Ӧ��ȡHCl |
| B����Ũ��ˮ����ʯ�ҷ�Ӧ��ȡNH3 |
| C����˫��ˮ��������̷�Ӧ��ȡO2 |
| D���ñ���ʳ��ˮ�͵�ʯ��Ӧ��ȡC2H2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��ȥCl2�л��е�����HCl���壬�ɽ�����ͨ��
| A����������Һ | B������NaHCO3��Һ | C������ʯ��ˮ | D�������Ȼ�����Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
̼��ƺ�����ƶ��ǸƵ���Ҫ��������������������ж����Ź㷺��Ӧ�á��ס�������ͬѧ�ֱ��̼��Ƶ��Ʊ�������Ƶ����ʽ���������̽����������벢��ɶ��й�����Ľ��
��1������ʹ�ô���ʯ(��������Fe2O3����)�������Ʊ�̼��Ƶ�ʵ���������£�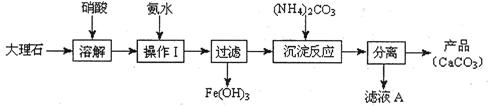
���ܽ����ʯʱ������������������ԭ���� ��
�����������У������롱�ò�Ʒ��������ʵ���������Ϊ�����ˡ� �� ��
�ۡ���ҺA���г�H+�����⣬�����е��������� ������������ӵ�ʵ�鷽���ǣ�ȡ������ҺA�� ���Թ��л�ϡ����ȳ�ַ�Ӧ����ʪ��ĺ�ɫʯ����ֽ(��pH��ֽ)�����Թܿڣ��۲����ɡ�
��2�������ij����ƾ���(xCaS04��yH20)���ȷֽ���йط�Ӧ����̽��������ȡ6.52g�þ�����м��ȣ����ȹ����У�����������ʱ��ı仯�������ͼ��ʾ����֪t5��t6ʱ����ڹ������������ԭ���Dz������������壬��Ӧ�Ļ�ѧ����ʽΪ��2CasO4 2CaO+2S02��+O2����
2CaO+2S02��+O2����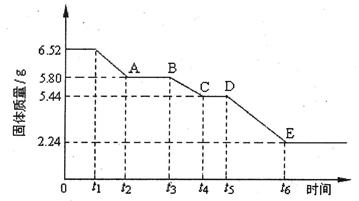
�ټ���ʱ���þ��忪ʼ������ѧ�仯��ʱ���� (�t1������t3����t5��)��
��t4��t5ʱ��ι���Ļ�ѧʽΪ ��
��tl��t2ʱ��ι��巢����Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ö������ȣ�ClO2���������ƣ�Na2FeO4Ħ������Ϊ166 g��mol��1�������;�ˮ�������ͳ�ľ�ˮ��Cl2�Ե�ˮ���������dz�������ˮ�����¼�����ClO2��Na2FeO4��ˮ���������зֱ𱻻�ԭΪCl����Fe3����
(1)����Ե�λ���������������õ��ĵ���������ʾ����Ч�ʣ���ôClO2��Na2FeO4��Cl2��������ɱ����������Ч���ɴ�С��˳���� �� �� �����ѧʽ��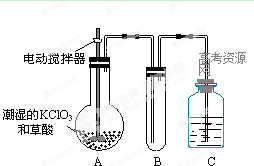
(2)����������һ�ֻ���ɫ�д̼�����ζ�����壬���۵�Ϊ��59�棬�е�Ϊ11.0�棬������ˮ����ҵ�����Գ�ʪ��KClO3�Ͳ�����60��ʱ��Ӧ�Ƶá�ijѧ��������ͼ��ʾ��װ��ģ�ҵ��ȡ���ռ�ClO2������AΪClO2�ķ���װ�ã�BΪClO2������װ�ã�CΪβ������װ�á����ʣ�
��A�з�Ӧ��ԭ���������������ʵ���֮��Ϊ1:2���÷�Ӧ�Ļ�ѧ����ʽΪ ��
A���ֻ�Ӧ�����¶ȿ��ƣ���ˮԡ���ȣ�װ�ã�B���ֻ�Ӧ����ʲôװ�ã� ��
�ڸ�װ�ð��ٲ���������װ��A��B��C�л���һ��������Բ��������� _
(�A��B��C��)��������
��C�е��Լ�ΪNaOH��Һ����Ӧʱ���������ƺ���������(NaClO2)�����÷�Ӧ�����ӷ���ʽΪ ����ʵ��ʱ��Ҫ450mL 4mol��L��NaOH��Һ�����ھ�ȷ����ʱ����Ҫ��ȡNaOH�������� g����ʹ�õ�������������ƽ����Ͳ����ͷ�ιܡ��������⣬�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�м���ʵ��С���ͬѧ��������ͼװ�ý��С�һ�����á���ʵ��̽��(a��ʢ�ŵ�Һ��������ٵ��£�b��ʢ�ŵ�ҩƷ����������c��d��ʢװҺ�壬���ܾ�����Һ������)��ÿ��ͬѧ������a��b��c��d�зֱ�ʢ�Ų�ͬ���ʣ�����ȡij�����岢���������ʡ�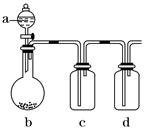
����ش����¸���ͬѧ�ڽ���ʵ����Ʒ�������������⣺
��.��1����a��Ũ���b���������(�������Աȶ�������ǿ�ܶ�)��c����ɫ�ʻ��ꡣ��ʵ�������c�е�����_____________________________________________________________��
dװ����ʢ��ҩƷ��������______________________________����д��d�з�Ӧ�����ӷ���ʽ�� ___________________________________________________________________��
��2����a��ϡ���b�����Ƿۣ�c������̼������Һ��d������Na2SiO3��Һ����ʵ���
���У�c�г��ֵ�������____________________��d��������____________����˵��
________________________________________________________________________��
��.����Ϊ����ͬѧ����ȡ����֮ǰ��Ӧ���еIJ�����________________���㻹�������ô�װ�õ�a��b��������ȡ��������(ֻдһ��)_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
Ϊ̽����ҵ����������ͭ�Ͻ���ϵ������ã���ͬѧ��Ƶ�ʵ�鷽�����£�
��ش�
��1���̷��Ļ�ѧʽΪ ��
��2��д����Ӧ�ٵĻ�ѧ����ʽ ��
��Ӧ�����ɳ��������ӷ�Ӧ����ʽ ��
��3��Ϊ�˼����ҺD�к��еĽ������ӣ������ʵ�鷽��Ϊ���Լ���ѡ���� ��
��4��������B�еμ�ϡ����ʱ�����ַ�Ӧ���ʱ�һ������۷�ӦҪ�죬��ԭ���� ��
��5����������ɫ��ѧ���գ�������E�м���ϡ������Լ�Y�Ƶ������壬�Լ�YΪ��ɫҺ�壬��Ӧ�ܵ��ܻ�ѧ����ʽΪ ������������ɫ��ѧ���գ���ѡ�Լ�YΪ1mol/L�����ᣬ��ʹ3molCuȫ���ܽ�����Һ�к�ͭԪ�ص����ʽ�ΪCuSO4��������������� L��
��ʯ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�����10�֣�
��������(NOCl)���л��ϳ��е���Ҫ�Լ�������NO��Cl2��ͨ�������·�Ӧ�õ�(NO2��Cl2��Ӧ�ɵ�������)���������ȵ��۵�Ϊ��64.5 �棬�е�Ϊ��5.5 �棬������ˮ�����ֽ�Ϊ������������Ȼ��⡣
ijУ��ѧ��ȤС�鰴���������Ʊ��������ȣ�ʵ��ʱ����ͨ��Cl2����E��U�ι��ڳ�������ɫ����ʱ���ٻ���ͨ��NO����������ʾ��������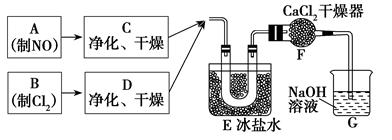
34��ʵ����������غ�Ũ�����Ʊ�Cl2�Ļ�ѧ����ʽ��______________________________��
35��װ��F��������___________________________________��
36������������ˮ��Ӧ�Ļ�ѧ����ʽ��________________________________��
37��ʵ���С���ͨ��Cl2����E��U�ι��ڳ�������ɫ����ʱ���ٽ�NO����ͨ�롱���˲�����Ŀ����______________________________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com