
��ҵ���������������ͼ���£���ش��������⣺

��1���������������Ի�����Ϊԭ�ϣ������ڹ����������������Ϊԭ�ϣ������� ��
��2��������������Ӧ��ǰ�辻����ԭ���� ��
��3���ڴ���Ӧ����ͨ��ʹ�ó�ѹ���ڴ�������SO2��ת����Ϊ90%�����Dz��ַ�����Ҳ�ȡ��ѹ��������ȡSO3����ȡ��ѹ��ʩ��Ŀ�ij��˼ӿ췴Ӧ�����⣬������ ���Ӷ��������Ч�ʡ�
��4����ҵ�����г��ð����ᷨ����β�������Դﵽ������Ⱦ���������õ�Ŀ�ġ��û�ѧ����ʽ��ʾ�䷴Ӧԭ���� ��
��5�������Ṥҵ�⣬�������ҵ������������صĹ�ҵ������������ȷ���� ��
A����ˮ���壺��ˮŨ�� ������
������ Һ��
Һ��
B����ˮ��þ����̲���� ʯ��ˮ
ʯ��ˮ MgO
MgO þ
þ
C����ҵ��������� NO2
NO2 ����������
����������
D����ҵ�ϳɰ�����Ȼ�� ����
���� NH3��H2��N2
NH3��H2��N2 ��
��
��15�֣���1���Ի�����Ϊԭ�ϵ������в����ķ�����̫�࣬�����ɱ��ߣ�2�֣�
��2����ֹ�����ж���2�֣� ��3��ʹƽ�����������ƶ������������SO2��ת���ʣ�2�֣�
��4��SO2+NH3+H2O��NH4HSO3��2�֣� 2NH4HSO3+H2SO4��(NH4)2SO4+2H2O+2SO2����3�֣�
��SO2+2NH3+H2O��(NH4)2SO3��2�֣� (NH4)2SO3+H2SO4��(NH4)2SO4+H2O+SO2����3�֣�
��5��A D����2�֣�
��������
�����������1��������������̫�࣬����Ի�����Ϊԭ�ϵ������в����ķ�����̫�࣬�����ɱ��ߡ�����ȼ�յIJ���ֻ��SO2�����ڴ�����������ڹ����������������Ϊԭ�ϡ�
��2���������ɵ������к��кܶ����ʣ�������ɴ����ж�������������������Ӧ��ǰ�辻����ԭ���Ƿ�ֹ�����ж���
��3�����ݷ���ʽ2SO2��O2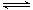 2SO3��֪���÷�Ӧ�������С�Ŀ��淴Ӧ���������ѹǿ����ʹƽ�����������ƶ������������SO2��ת���ʡ�
2SO3��֪���÷�Ӧ�������С�Ŀ��淴Ӧ���������ѹǿ����ʹƽ�����������ƶ������������SO2��ת���ʡ�
��4�������Ǽ������壬SO2���������壬��˰�����������SO2������������炙�������李����ɵ���������炙������������ϡ���ᷴӦ��ת��ΪSO2���Ӷ��ﵽĿ�ġ��йط�Ӧ�ķ���ʽΪSO2+NH3+H2O��NH4HSO3��2NH4HSO3+H2SO4��(NH4)2SO4+2H2O+2SO2����SO2+2NH3+H2O��(NH4)2SO3��(NH4)2SO3+H2SO4��(NH4)2SO4+H2O+SO2����
��5��A���������������ԣ������������������ɵ����壬Ȼ��������SO2�����������Դﵽ������Ŀ�ġ���������������������Խ������ӻ�ԭ���ɵ����壬���ѡ��A����ʵ�ֺ�ˮ���壬A��ȷ��B����ҵ����ͨ��������ڵ��Ȼ�þ��ұ������þ�������������۵�̫�ߣ���Ҫ���Ĵ�����Դ��B����ȷ��C�������еĵ����ڷŵ������������������ֻ������NO���ò���NO2��C����ȷ��D�������ȵ���Ҫ�ɷ��Ǽ��飬��һ�������¿����������������뵪�����Ժϳɰ��������ѡ��D����ʵ�ֹ�ҵ�ϳɰ���D��ȷ����ѡAD��
���㣺���鹤ҵ�Ʊ�������й��ж�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

��ش��������⣺
��1��д��FeS2��������Ӧ�Ļ�ѧ����ʽ��____________________________��
��2������װ���г�����������__________________________________________��
��3��д����ͼ��װ�õ�����__________________________________________��
��4����װ���ҵķ�Ӧ����Ҫ����������Ҫ�ȶ������������һ�����ң�ʵ��ʱ������ο�������Ƶģ�____________________________��
��5���Ӵ�������������У�����Ӧ�ȶ�δ�����ã���ÿ����1 ![]() 2SO3��g������H=-98.3 kJ��mol-1�������������������еõ�������ã�������Ӧ�Ȳ��ƣ�����ÿ����1
2SO3��g������H=-98.3 kJ��mol-1�������������������еõ�������ã�������Ӧ�Ȳ��ƣ�����ÿ����1
��6����ʵ�����Ӷ�װ�õ�Ŀ����Ϊ��̽��____________________________��
��7����ʵ����ƻ����ڵĽ�����ȱ����_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
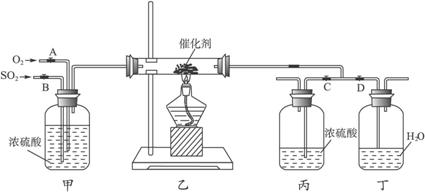
��ش��������⣺
(1)д��FeS2��������Ӧ�Ļ�ѧ����ʽ��_______________��
(2)����װ���г�����������_______________��
(3)д����ͼ��װ�õ�����______________________________��
(4)��װ���ҵķ�Ӧ����Ҫ����������Ҫ�ȶ������������һ�����ң�ʵ��ʱ������ο�������Ƶģ�_______________��
(5)�Ӵ�������������У�����Ӧ�ȶ�δ�����ã���ÿ����1 ![]() 2SO3(g)����H=-98.3 kJ��mol-1�������������������еõ��������(������Ӧ�Ȳ���)����ÿ����1
2SO3(g)����H=-98.3 kJ��mol-1�������������������еõ��������(������Ӧ�Ȳ���)����ÿ����1
(6)��ʵ�����Ӷ�װ�õ�Ŀ����Ϊ��̽��______________________________��
(7)��ʵ����ƻ����ڵĽ�����ȱ����______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ģ���� ���ͣ������
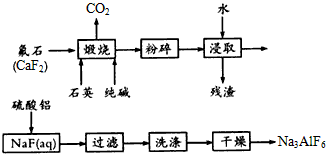
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����װ���Ƿ��չ�ҵ���Ʊ������������Ƴ����ġ�
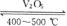
��1��д������������������Ӧ�ķ���ʽ��_______________________________________��
��2��ͼ�мס��ҡ�������װ�÷ֱ��൱�ڹ�ҵ������ȡ�����е�_____________________��
��3��д���ڴ��������������ķ�Ӧ�ķ���ʽ��___________________________________��
��4�����ҷ�Ӧ����Ҫ����������Ҫ�ȶ������������һ�����ң�����ο�������ƣ�
__________________________________________________________��
��5��ѧ��Ϊ��̽����ҵ��Ϊ�β���98.3%��Ũ���������������������ˮ����������װ�ý���ģ��ʵ�顣����װ���ڷ�Ӧ�������ȳ������ݣ����þͳ����˰���������װ��һֱ��û���κ����������������ԭ���ǣ�_______________������β���ķ����У�__________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com