
(12��) ����ʯ����Ҫ�ɷ���K2SO4��Al2(SO4)3��2Al 2O3��6H2O�������������Fe2O3���ʡ���������ʯ�Ʊ������������������£�

�ش��������⣺
��1�������ա������з����ķ�ӦΪ2Al2(SO4)3��3S  2Al 2O3 ��9SO2 ������������Ϊ ��
2Al 2O3 ��9SO2 ������������Ϊ ��
��2�����ܽ⡱ʱ��Ӧ�����ӷ���ʽΪ ��
��3��������pH������ˡ�ϴ��Al(OH)3������֤����ϴ�Ӹɾ���ʵ�������������
��
��4������pHʱʹ�õ�������Ũ�ȣ���λ�����Һ�������ʵ�������Ϊ882 g/L ��H2SO4 ������1L����Һ��������Ͳ��ȡ��������Ϊ98�������ᣨ�ܶ���1.8g/cm3��
mL
��5��������������ĸҺ���пɻ��յ����ʷֱ��� ��
 һ����������ϵ�д�
һ����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(12��) ����ʯ����Ҫ�ɷ���K2SO4��Al2(SO4)3��2Al2O3��6H2O�������������Fe2O3���ʡ���������ʯ�Ʊ������������������£�
�ش��������⣺
��1�������ա������з����ķ�ӦΪ2Al2(SO4)3��3S 2Al 2O3 ��9SO2 ������������Ϊ ��
��2�����ܽ⡱ʱ��Ӧ�����ӷ���ʽΪ ��
��3��������pH������ˡ�ϴ��Al(OH)3������֤����ϴ�Ӹɾ���ʵ�������������
��
��4������pHʱʹ�õ�������Ũ�ȣ���λ�����Һ�������ʵ�������Ϊ882 g/L ��H2SO4 ������1L����Һ��������Ͳ��ȡ��������Ϊ98�������ᣨ�ܶ���1.8g/cm3��
mL
��5��������������ĸҺ���пɻ��յ����ʷֱ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ��ͨ��ͨ���������ص��ȵ�ר���⻯ѧ�Ծ� ���ͣ������
(12��) ����ʯ����Ҫ�ɷ���K2SO4��Al2(SO4)3��2Al 2O3��6H2O�������������Fe2O3���ʡ���������ʯ�Ʊ������������������£�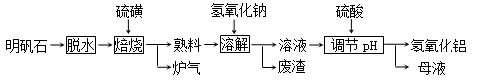
�ش��������⣺
��1�������ա������з����ķ�ӦΪ2Al2(SO4)3��3S  2Al 2O3��9SO2������������Ϊ ��
2Al 2O3��9SO2������������Ϊ ��
��2�����ܽ⡱ʱ��Ӧ�����ӷ���ʽΪ ��
��3��������pH������ˡ�ϴ��Al(OH)3������֤����ϴ�Ӹɾ���ʵ�������������
��
��4������pHʱʹ�õ�������Ũ�ȣ���λ�����Һ�������ʵ�������Ϊ882 g/L ��H2SO4 ������1L����Һ��������Ͳ��ȡ��������Ϊ98�������ᣨ�ܶ���1.8g/cm3��
mL
��5��������������ĸҺ���пɻ��յ����ʷֱ��� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com