



 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
|
| ||
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

(1)���д���Ϊ99.5%���Ҵ�������ȥ���е�ˮ�֣��ô����Ҵ������д�ʩ��������( )
A.�����ռ���һ��� B.��CuSO4��H2O��ϣ����ˣ��ռ���Һ
C.��CaO��H2O��Ӧ�����ˣ��ռ���Һ D.��CaO��H2O��Ӧ�������ռ����
(2)Ԥ�ⷴӦ�����п�����380 mL H2������ʵ�װ������˳����________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
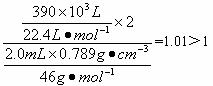
��ش��������⣺
(1)���������������ȷװ����___________(��д���)��
(2)װ����A���ֵķ�Һ©����������ƿ֮�����ӵĵ��������������___________(��д���)��
A.��ֹ��ˮ�ƾ��ӷ� B.��֤ʵ��װ�ò�©�� C.ʹ��ˮ�ƾ�������
(3)ʵ��ǰԤ�Ƚ�С�����ڶ��ױ����ۻ���С���飬��ȴ������ƿ�У���Ŀ����____________________________________________��
(4)��֪��ˮ�ƾ����ܶ�Ϊ
(5)ʵ�����ⶨ�Ľ��ƫ�ߣ����������ԭ����______________________(��д���)��
A.��ʵ���������½��� B.��ˮ�ƾ��л������״� C.��ˮ�ƾ����Ƶķ�Ӧ������ȫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������⣺
(1)���������������ȷװ����____________(��д���)��
(2)װ����A���ֵķ�Һ©����������ƿ֮�����ӵĵ��������������____________(��д���)��
A.��ֹ��ˮ�ƾ��ӷ�
B.��֤ʵ��װ�ò�©��
C.ʹ��ˮ�ƾ�������
(3)ʵ��ǰԤ�Ƚ�С�����ڶ��ױ����ۻ���С���飬��ȴ������ƿ�У���Ŀ����________________________________________________________________��
(4)��֪��ˮ�ƾ����ܶ�Ϊ
(5)ʵ�����ⶨ�Ľ��ƫ�ߣ����������ԭ����____________(��д���)��
A.��ʵ���������½���
B.��ˮ�ƾ��л������״�
C.��ˮ�ƾ����Ƶķ�Ӧ������ȫ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com