
��10�֣�2010���Ϻ������������ǡ����У�����������á����Իش��������⣺
��1��Ϊȷ���������ڼ���ǿ������������ʴﵽ95%���ϣ��������ڼ�Ŀ�������״������У�����Ҫ����ָ����_____________��
a������������PM10�� b��NO2Ũ�� c��SO2Ũ�� d��CO2Ũ�ȣ�
��2����ɳ�����ն�����Ҫ������_______________��Ϊ�˸��ƿ���������������ƴ����ж�������������̳�����Ⱦ����ŷ�������������װβ��������װ�ã�ʹ���е��к�����NO��COת��Ϊ�����壬�÷�Ӧ�Ļ�ѧ����ʽΪ__________��
��3��Ϊ2010���������ڼ��ṩ��ѧ��Ч�Ļ����������Ϸ�������������ˮ�������������죬������ˮʱ����K2SO4��Al2(SO4)3��24H2O���ʽ�Ȼ�����������____________��ͨ�������������ȵ�������______________��ijũ�����Ϊ�������ˮ���ڽ��ر�ˮȡ�ؼҺ�ʹ��Ư�ۻ�Ư��Ƭ����ɱ����������ԭ�����û�ѧ����ʽ��ʾΪ_________________________��
��4���������й��ݡ���������֮�ڡ������ɸֽ��������7000����ɫ�����1200��鲣���Ƚ��ɡ������������õĹ�ҵ�豸�� ��ʯӢ������ѧ�ȶ���ǿ������ϵ��С����һ�����ֲ�����ʯӢ��������Ҫ�ɷ��� ��
 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 2010���Ϻ������᳡�ݣ��������������ϻ���Ļ��ʹ���˷�������ܣ�LED����Ŀǰ����LEDƷƬ�����ʻ�����GaAs���黯�أ���AlGaInP��������������lnGaN���������أ�Ϊ������֪������ͬ����һ���ڵ�Ԫ�أ��黯�صľ����ṹ��ͼ���Իش�
2010���Ϻ������᳡�ݣ��������������ϻ���Ļ��ʹ���˷�������ܣ�LED����Ŀǰ����LEDƷƬ�����ʻ�����GaAs���黯�أ���AlGaInP��������������lnGaN���������أ�Ϊ������֪������ͬ����һ���ڵ�Ԫ�أ��黯�صľ����ṹ��ͼ���Իش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 2010���Ϻ������᳡�ݴ������������ϻ���Ļ��ʹ���˷�������ܣ�LED����Ŀǰ����LED��Ƭ���ʻ�����GaAs���黯�أ���AlGaInP��������������InGaN���������أ�Ϊ������֪������ͬ����һ���ڵ�Ԫ�أ��黯�صľ����ṹ��ͼ���Իش�
2010���Ϻ������᳡�ݴ������������ϻ���Ļ��ʹ���˷�������ܣ�LED����Ŀǰ����LED��Ƭ���ʻ�����GaAs���黯�أ���AlGaInP��������������InGaN���������أ�Ϊ������֪������ͬ����һ���ڵ�Ԫ�أ��黯�صľ����ṹ��ͼ���Իش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
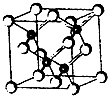 ��2011?�Ͼ�ģ�⣩2010���Ϻ������᳡�ݣ��������������ϻ���Ļ��ʹ���˷�������ܣ�LED����Ŀǰ����LEDƷƬ�����ʻ�����GaAs���黯�أ���AlGaInP��������������InGaN���������أ�Ϊ�����黯�ص�Ʒ���ṹ��ͼ���Իش�
��2011?�Ͼ�ģ�⣩2010���Ϻ������᳡�ݣ��������������ϻ���Ļ��ʹ���˷�������ܣ�LED����Ŀǰ����LEDƷƬ�����ʻ�����GaAs���黯�أ���AlGaInP��������������InGaN���������أ�Ϊ�����黯�ص�Ʒ���ṹ��ͼ���Իش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com