
N2(g)+3H2(g)![]() 2NH3(g)
2NH3(g)
(1)����Ӧ���е�ijʱ��tʱ��ni(N2)=13mol��ni(NH3)=6mol������a��ֵ.
(2)��Ӧ�ﵽƽ��ʱ�������������Ϊ726.8L(�����)������NH3�ĺ���(�������)Ϊ25%.����ƽ��ʱNH3�����ʵ���.
(3)ԭ���������ƽ��������������ʵ���֮��(д����������ȣ���ͬ)��n(ʼ)��n(ƽ)=________.
(4)ԭ������壬a:b= ________.
(5)�ﵽƽ��ʱ��N2��H2��ת����֮�ȣ�a(N2):a(H2)=________.
(6)ƽ���������У�n(N2):n(H2):n(NH3)=________.
| (1)��=16 (2)ƽ��ʱn��NH3��=8mol (3)5:4 (4)2:3 (5)1:2 (6)3:3:2
|
| (1)˼·һ���ɷ�Ӧ�Ļ�ѧ����ʽ��֪�����ĵ�N2�����ɵ�NH3֮�ȵ��ڻ�ѧ����ʽ�ļ���ϵ��֮�ȣ������ĵ�N2�����ʵ���Ϊxmol����x:6��1:2��x=3���߷�Ӧ�����ʼ��=ijʱ�����仯������a=13��3=16
˼·����
N2��3H2 ��ʼ��(mol) a b 0 tʱ��(mol) 13 6 �仯��(mol) 3 6 ��a=13+3=16mol (2)˼·�������������Ϊƽ��ʱ���������ʵ���������
˼·���� N2��3H2 ��ʼ����(mol) a b 0 �仯��(mol) x 3x 2x ƽ����(mol) ����x b��3x 2x
��nƽ(����)=2x=8mol (3) (5)��ij��Ӧ���ƽ��ʱ��ת����= ��ﵽƽ��ʱ��N2��H2��ת����֮��=��(N2):��(H2) = (6)ƽ���������У�n(N2)��n(H2):n(NH3)=(����x):(b��3x):2x�����Ц�=16mol��b=24mol����n(N2):n(H2):n(NH3)=12:12:8=3:3:2.
|


 �ľ�ͼ���ʱ�ȷ�ϵ�д�
�ľ�ͼ���ʱ�ȷ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 2NH3��g��
2NH3��g���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009-2010ѧ��㶫ʡ����������������ѧ�������ϣ��¿���ѧ�Ծ��������棩 ���ͣ������
 2NH3��g��
2NH3��g���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ר���� ���ͣ������
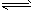 2NH3��g��
2NH3��g���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£���amolN2��bmolH2�Ļ������ͨ��һ���̶��ݻ����ܱ������У��������·�Ӧ��N2(g)+3H2(g)![]() 2NH3(g)
2NH3(g)
(1)����Ӧ���е�ijʱ��tʱn1(N2)=13mol,n1(NH3)=6moL,����a= ��
��2����Ӧ��ƽ��ʱ�������������Ϊ716.8L������£�������NH3���������Ϊ25%����ƽ��ʱNH3�����ʵ���Ϊ ��
(3)ԭ���������ƽ��������������ʵ���֮��Ϊ��д�����������,��ͬ�� ��
��4��ԭ��������У�a:b= ��
��5����ƽ��ʱ��N2��H2��ת����֮��Ϊ ��
��6��ƽ���������У�n(N2):n(H2):n(NH3)= ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com