
 ��֪25��ʱ����������ʵĵ���ƽ�ⳣ�����������
��֪25��ʱ����������ʵĵ���ƽ�ⳣ�����������| ��ѧʽ | CH3COOH | H2CO3 | HClO | |
| ƽ�ⳣ�� | Ka=1.8��10-5 | Ka1=4.3��10-7 | Ka2=5.6��10-11 | Ka=3.0��10-8 |
���� ��1������ĵ���ƽ�ⳣ��Խ��������Խǿ���������ˮ��̶�Խ����
��2��0.1mol/L��CH3COOH��Һ��ˮϡ�����У��������������������ʵ���������������ʵ�����С��Ũ�ȼ�С�����Լ�����ˮ�����ӻ��������䣬����ĵ���ƽ�ⳣ�����䣻
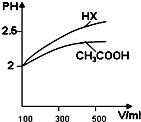
��3����ͼ��������ˮϡ�͵Ĺ����У�HX��pH�仯�ȽϿ죬˵��HX�����Աȴ���ǿ��
��4��25��ʱ��CH3COOH��CH3COONa�Ļ����Һ�������pH=6����Һ�����ԣ�����ĵ���̶ȴ��ڴ�������ӵ�ˮ��̶ȣ�����Ka=$\frac{c��C{H}_{3}CO{O}^{-}��•c��{H}^{+}��}{c��C{H}_{3}COOH��}$����
��� �⣺��1���ݵ���ƽ�ⳣ����֪��������ǿ������˳��Ϊ��CH3COOH��H2CO3��HClO��HCO3-�����������Խ����������ӵ�ˮ��̶�Խ����Һ����Խǿ������pH��С��������˳����a��d��c��b��
�ʴ�Ϊ��a��d��c��b��
��2��0.1mol/L��CH3COOH��Һ��ˮϡ�����У��������������������ʵ�������Ũ�ȼ�С�����Լ�����
A��������Ũ�ȼ�С���ʴ���
B����ˮϡ�����У����������ʵ���������������ʵ�����С������$\frac{c��{H}^{+}��}{c��C{H}_{3}COOH��}$������ȷ��
C��ˮ�����ӻ��������䣬�ʴ���
D��������Һ��ˮϡ��ʱ���Լ�����������Ũ�ȼ�С����������Ũ����������$\frac{c��O{H}^{-}��}{c��{H}^{+}��}$������ȷ��
E��$\frac{c��{H}^{+}��•c��C{H}_{3}CO{O}^{-}��}{c��C{H}_{3}COOH��}$�Ǵ���ĵ��볣��������ĵ���ƽ�ⳣ�����䣬�ʴ���
�ʴ�Ϊ��BD��
��3����ͼ��������ˮϡ�͵Ĺ����У�HX��pH�仯�ȽϿ죬˵��HX�����Աȴ���ǿ��HX�ĵ���ƽ�ⳣ���ȴ����
�ʴ�Ϊ������
��4��25��ʱ��CH3COOH��CH3COONa�Ļ����Һ�������pH=6����Һ�����ԣ�����ĵ���̶ȴ��ڴ�������ӵ�ˮ��̶ȣ�����Һ������Ũ�ȴ�С��ϵΪ��c��CH3COO-����c��Na+����c��H+����c��OH-������֪Ka=$\frac{c��C{H}_{3}CO{O}^{-}��•c��{H}^{+}��}{c��C{H}_{3}COOH��}$=1.8��10-5����֪pH=6����c��H+��=10-6mol/L������$\frac{c��C{H}_{3}CO{O}^{-}��}{c��C{H}_{3}COOH��}$=18��
�ʴ�Ϊ��c��CH3COO-����c��Na+����c��H+����c��OH-����18��
���� ���⿼��������ʵĵ��룬����ƽ�ⳣ��ȷ������ǿ�����Ӷ�ȷ��ˮ��̶ȣ�ע���ˮϡ��ʱ�Ӹ��������ʵ����仯�������Ѷ��еȣ������ڿ���ѧ���ķ��������ͶԻ���֪ʶ��Ӧ��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �÷�Ӧ����������C12 | B�� | ÿ��1mol C12��Ӧת��1mole- | ||
| C�� | ��Ӧ��SԪ�صļ�̬���� | D�� | �÷�Ӧ��H2O������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �÷�Ӧ�Ļ�ѧ����ʽΪ2H2CrO4+3H2O2=2Cr��OH��3+3O2��+2H2O | |
| B�� | �÷�Ӧ�е���������H2O2����ԭ������O2 | |
| C�� | �����ԣ�H2CrO4��O2 | |
| D�� | �練Ӧת����0.3 rnol���ӣ�������������ڱ�״�������Ϊ3.36 L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1mol CO��g����1molH2O��g����ַ�Ӧ���ų�������ΪaKJ | |
| B�� | �ı�ѹǿ��ƽ�ⲻ�����ƶ�����Ӧ�ų����������� | |
| C�� | �����¶ȣ����ʼӿ죬��Ӧ�ų����������� | |
| D�� | �����÷�Ӧ���Ϊԭ��أ���Ӧ�ų����������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 18O 31P 35Cl | B�� | 27Al 19F 12C | ||
| C�� | ��һ��������Ԫ�ص�ԭ�� | D�� | Ԫ�����ڱ��Т�A����Ԫ�ص�ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ԭ��ص������������������ֲ�ͬ�Ľ��� | |
| B�� | �κλ�ѧ��ӦֻҪ���Է����еķ��ȷ�Ӧ�����Ա���Ƴ�ԭ��� | |
| C�� | ��ԭ����У�����������һ���Ǹ������õ缫����ԭ | |
| D�� | ԭ����ǻ�ѧ��ת��Ϊ���ܵ�װ�ã�Ϊ����ṩ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ij��Һ�д��ڵ�����ֻ��Cl-��OH-��NH4+��H+������Һ������Ũ�ȴ�С��ϵ����Ϊ��c��Cl-����c��NH4+����c��OH-����c��H+�� | |
| B�� | 0.1mol•L-1��NH4��2SO4��Һ�У�c��H+����c��NH4+�� | |
| C�� | 0.1mol•L-1��HCl��Һ��0.1mol•L-1��NaOH��Һ�������ϣ�c��H+��+c��Na+���Tc��OH-��+c��Cl-�� | |
| D�� | 0.1mol•L-1 CH3COONa��Һ��c��Na+����c��CH3COO-����c��H+����c��OH-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com