
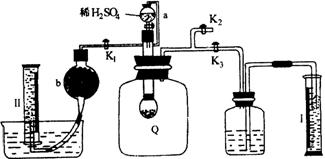
Ϊ�˲ⶨ�Ѳ��ֱ��ʵĹ���������Ʒ�Ĵ��ȣ������ͼ��ʾ��ʵ��װ�ã�ͼ��QΪ�������õ�����ȡһ��������Ʒ�������У���ͼ��װ������������©���Ļ�������ϡ������������С�����գ�
|
��1��Q�ڷ�����ѧ��Ӧʱ�����ɵ������� ��
��2��Ϊ�˲ⶨ��Ӧ�����������������μ�ϡ����֮ǰ��K1��K2��K3��Ӧ���رյ��� ��Ӧ������ ��
��3����������Ӧֹͣ����K1��K2��K3�����ڹر�״̬��Ȼ���ȴ�K2���ٻ�����K1����ʱ�ɹ۲쵽�������� ��
��4��b��װ�Ĺ����Լ��� ��ΪʲôҪ������K1 ��
��5��ʵ�����ʱ����ͲI����x mLˮ����ͲII���ռ���y mL���壨������ɱ�״̬������������ƵĴ��ȵ���ѧ����ʽ�� ���軯��������ʽ��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2013?բ������ģ��������Ļ������ڹ�ҵ������Ӧ�ù㷺���ش��������⣺
��2013?բ������ģ��������Ļ������ڹ�ҵ������Ӧ�ù㷺���ش��������⣺| װ�� | A ���Թ�+��ĩ�� |
B | C |
| ��Ӧǰ | 42.0g | 75.0g | 140.0g |
| ��Ӧ�� | 37.0g | 79.0g | 140.5g |
| ���� |
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʦ����2006��2007ѧ�����ѧ�ڸ����꼶�¿�(��)����ѧ ���ͣ�058
| |||||||||||||||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

ͼ15-11
��1��Q�ڷ�����ѧ��Ӧʱ�����ɵ�������_____________��
��2��Ϊ�˲ⶨ��Ӧ�����������������μ�ϡ����֮ǰ��K1��K2��K3��Ӧ���رյ���__________��Ӧ������_____________��
��3����������Ӧֹͣ����K1��K2��K3�����ڹر�״̬��Ȼ���ȴ�K2���ٻ�����K1����ʱ�ɹ۲쵽��������__________________________________________________________��
��4��b��װ�Ĺ����Լ���___________��ΪʲôҪ������K1__________________________��
��5��ʵ�����ʱ����Ͳ������x mLˮ����Ͳ�����ռ���y mL���壨������ɱ�״��������������ƵĴ��ȵ���ѧ����ʽ��__________________________���軯��������ʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)Q�ڷ�����ѧ��Ӧʱ�����ɵ�������__________________________________________��
(2)Ϊ�˲ⶨ��Ӧ�����������������μ�ϡ����֮ǰ��K1��K2��K3��Ӧ���رյ���_______________��Ӧ������_______________��
(3)��������Ӧֹͣ����K1��K2��K3�����ڹر�״̬��Ȼ���ȴ�K2���ٻ�����K1����ʱ�ɹ۲쵽��������__________________________________________��
(4)b��װ�Ĺ����Լ���___________________________________________��ΪʲôҪ������K1?___________________________________________________________��
(5)ʵ�����ʱ����Ͳ������x mLˮ����������ռ���y mL����(������ɱ�״̬)����������ƵĴ��ȵ���ѧ����ʽ��___________________________(����������ʽ)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com