
| A��5.85gNaCl��������100gˮ��������Һ���ʵ���Ũ�Ȳ�����1mol/L |
| B��20gŨ��Ϊc mol��L-1�İ�ˮ�м���һ������ˮϡ�ͳ�0.5cmol��L-1�������ˮ�����С��20ml |
| C��ij�Ȼ�þ��Һ���ܶ�Ϊ1.18 g��cm��3������þ���ӵ���������Ϊ5.1%,300 mL����Һ��Cl�������ʵ���Լ����1.50 mol |
D��V L Fe2(SO4)3��Һ�к�Fe3��m g������Һ��SO42-�����ʵ���Ũ��Ϊ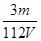 mol��L��1 mol��L��1 |
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
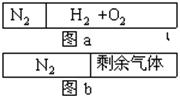
| A��1�U1 | B��1�U3 | C��7�U1 | D��5�U1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��1 Lˮ���ܽ���58.5 g NaCl������Һ�����ʵ���Ũ��Ϊ1 mol/L |
| B����1 L 2 mol/L��H2SO4��Һ��ȡ��0.5 L������Һ��Ũ��Ϊ1 mol/L |
| C������500 mL 0.5 mol/L��CuSO4��Һ����62.5 g���� |
| D���к�100 mL 1 mol/L��H2SO4��Һ����NaOHΪ4 g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ͬ��ͬѹ�¼��飨CH4�����������ܶ�֮��Ϊ2��1 |
| B��1 g�����1 g������ԭ����֮��Ϊ5��1 |
| C�������ʵ����ļ��������������֮��Ϊ2��1 |
| D���ڱ�״���µ������ļ�������������֮��Ϊ1��2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A������������������룻 |
| B����NaOH����ֽ���ϳ����� |
| C��NaOH���ձ����ܽ��δ��ȴ������ת�Ƶ�����ƿ�У� |
| D������ʱ���ӿ̶��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��2g��������ԭ������NA |
| B��17g NH3�����ĵ�������10NA |
| C��NA�������Ӻ�NA������ӵ������ȵ���1��1 |
| D��2.3g������ȫ�����������ʱʧȥ�ĵ�������0.2NA |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com