��֪A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵ�ԭ������������������A��Cԭ�ӵ�L����2��δ�ɶԵ��ӣ�D��Eͬ���壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��F
3+����M��3d�������Ϊ����״̬�����������������ش��������⣺������ʱ��������Ӧ��Ԫ�ط��ű�ʾ��
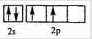
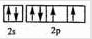
��1��д��Cԭ�ӵļ۲�����Ų�ͼ______��Fλ�����ڱ�______����
��2��A��B��C�ĵ�һ��������С�����˳��Ϊ______����дԪ�ط��ţ�
��3��F��������Ϊ25��M�IJ��ֵ��������������±�
| Ԫ�� |
M |
F |
| �����ܣ�kJ?mol-1�� |
I1 |
717 |
759 |
| I2 |
1509 |
1561 |
| I3 |
3248 |
2957 |
�Ƚ���Ԫ�ص�I
2��I
3��֪����̬M
2+��ʧȥһ�����ӱ���̬F
2+��ʧȥһ�������ѣ��Դˣ���Ľ�����______��
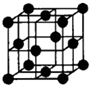
��4��������Fԭ�ӵ���λ��Ϊ______����Fԭ�ӵİ뾶Ϊrcm����F������ܶ�Ϊ______���ú�r�ı���ʽ��ʾ�����þ�����ԭ�ӿռ�������Ϊ______��
��5��H
2S��CԪ�ص��⻯�����ʽΪH
2C
2����Ҫ�������ʱȽ�����
|
�۵�/K |
�е�/K |
��״��ʱ��ˮ�е��ܽ�� |
| H2S |
187 |
202 |
2.6 |
| H2C2 |
272 |
423 |
������Ȼ��� |
H
2S��H
2C
2����Է�������������ͬ����������������ʲ������Ҫԭ��______��





 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д� �����Ծ���Ԫ���Ծ�ϵ�д�
�����Ծ���Ԫ���Ծ�ϵ�д�


 ��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ�
��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ� ����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�
����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�