

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
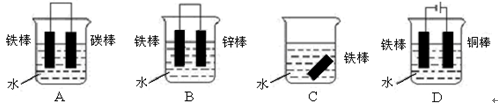
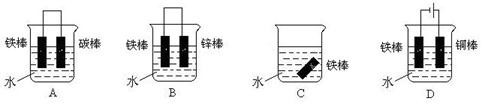
| A�����뺣�ڵĸ���բ����װһ��������п��ɷ�ֹբ�ű���ʴ |
| B���ֹ����Դ�������ӣ��ֹܿɱ����� |
| C���ֹ���ͭ��¶��ѷ���һ�𣬼��ٸֹܸ�ʴ |
| D�������������ⸯʴʱ��������Ӧ��Fe�� Fe3++3e- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
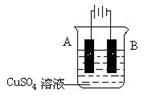
| A���������Ľ������Դ���������� |
| B���������Ľ������Դ�ĸ������� |
| C���ڱ������Ľ��������Ϸ���������Ӧ |
| D���������Ľ��������ϲ�����������Ӧ��Ҳ��������ԭ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
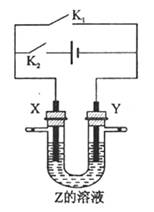
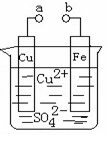
| A�����ر�K2,��K1��X��Y��Ϊʯī,Z��NaCl,��X�����ܵõ��������� |
| B�����ر�K2����K1,X�Ǵ�ͭ,Y�Ǵ�ͭ,Z��CuSO4,��װ�ÿ�����ͭ������ |
C�����ر�K1����K2,X��Cuͭ��Y����H2SO4������Һ�е� ����Y�� ����Y�� |
| D�����ر�K1,��K2��X��Cuͭ,Y��Fe��Z�Ǻ��п����ĺ�ˮ����װ�ÿ����ڱ���Fe |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ����ȫ���գ��Ƶ��н�ǿɱ������������Һ(��84����Һ��)���������ͼ��װ�ã���Ե�Դ�缫���ƺ�����Һ����Ҫ�ɷ��ж���ȷ����
����ȫ���գ��Ƶ��н�ǿɱ������������Һ(��84����Һ��)���������ͼ��װ�ã���Ե�Դ�缫���ƺ�����Һ����Ҫ�ɷ��ж���ȷ����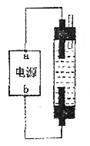
| A��a������b������NaClO��NaCl |
| B��a������b������NaClO��NaCl |
| C��a������b������HClO��NaCl |
| D��a����'b������HClO��NaCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ͭƬ | B��̼�� | C��пƬ | D����Ƭ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com