
ZnCO3�������Һ��Ӧ���������壬�����ȷ����ؽᾧ���õ���ˮ����A��A�Ǿ��кܸ߶Գ��Ե��������ӡ���A�ڿ��������գ��õ�ZnO��ʧ��48.4%��
1��ͨ������ȷ��A�Ļ�ѧʽ��
2��д���Ʊ�A�Ļ�ѧ����ʽ��
3��Zn�ij�����λ����4������A���ӵĿռ�ṹ��˵������ԭ��Zn���ӻ�̬��
4�����п�ֱ�Ϊ��A����B��Ԫ�أ��ںܶ������������ơ����BeҲ�����γɽṹ��A���Ƶ������B����Zn���������ˮ�⣬��Be������ﲻ��ˮ�⡣����͡�
1�����A������ֻ��1��Znԭ�ӣ���һ����ΪZnAc2��183.4������ʽ��Ϊ157.8��������ΪZnAc2���ұ�ZnAc2С25.6����1�֣�
��A�Ļ�ѧʽΪZnOxAc2��2x����Zn(OH)yAc2��y����x��0.25��y��0.61����������������A�Ļ�ѧʽΪZn4OAc6��2�֣�
2��4BeCO3��6CH3COOH��4CO2����3H2O��Be4O(OOCCH3)6��2�֣�
3��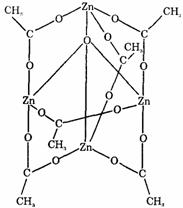 ��2�֣� sp3��1�֣�
��2�֣� sp3��1�֣�
4����ΪBe�ǵڶ�����Ԫ�أ�Be��sp3�ӻ��ɼ����ĸ��۹���ѱ��ͣ���d�����H2O������Oԭ�ӵĵ������Խ�������Be4O(CH3COO)6����ˮ�⡣��Zn���ڵ������ڣ���d�����H2O���ӿ��Խ���Zn��d���������Zn4O(CH3COO)6����ˮ�⡣��2�֣�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com