
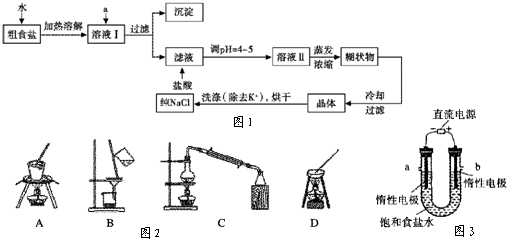
2- 4 |
2- 4 |
| ��ѧʽ | CaCO3 | CaSO4 | Ca��OH��2 | MgCO3 | Mg��OH��2 |
| Ksp | 2.8��10-9 | 9.1��10-6 | 1.0��10-4 | 6.8��10-6 | 1.8��10-11 |
| ||



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1��ը����ʱ1 kg��������0.5 kgˮ��4 g������10 gС�մ�����ʳ�εȸ��ϣ�����ը����Ʒ�����IJ���һ��Ϊ80%��ͨ������˵����ÿ��ʳ��100 g��������������������__________________��?
��2�����о��ҹ��������ճ�����������������ʳƷ���Ӽ��⣩�����ֿ���;����______________________________________________________________________?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0115 �¿��� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com