



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��12�֣��ϳɰ����������Ĵ����������˹��̵���;�����Ի�ѧ��ҵ����Ҳ�������ش�Ӱ�졣�ϳɰ���Ӧ�Ļ�ѧ����ʽΪ��N2(g)��3H2(g)![]() 3NH3(g)��H����92.2kJ/mol���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2����̼�ڸ�������ˮ������Ӧ�Ƶá��ҹ��ϳɰ���ҵĿǰ����������Ϊ������������ý���¶ȣ�400��500�棬ѹǿ��30��50MPa���ش��������⣺
3NH3(g)��H����92.2kJ/mol���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2����̼�ڸ�������ˮ������Ӧ�Ƶá��ҹ��ϳɰ���ҵĿǰ����������Ϊ������������ý���¶ȣ�400��500�棬ѹǿ��30��50MPa���ش��������⣺
��1����ˮ����ͨ�����ȵ�̿������ˮú����
C(s) + H2O(g) H2(g) +CO(g) ��H = +131.3 kJ ����S = +133.7 J/K
�÷�Ӧ�ڵ������ܷ��Է� ����ܻ��
��2���ϳɰ���ҵ��ԭ����ѹ����30��50MPa��ԭ���� ����ƽ���ƶ�ԭ������������������ԭ������ת����ʵ�������в���400��500��ĸ��£�ԭ��֮һ�ǿ��ǵ������Ĵ����ԣ�ԭ��֮���� ��
��3����֪��400��ʱ��N2 (g)+ 3H2(g) 2NH3(g) ��K=0.5��
����400��ʱ�� 2NH3(g)N2 (g)+ 3H2(g)��K= ������ֵ����
��400��ʱ����0.5L�ķ�Ӧ�����н��кϳɰ���Ӧ��һ��ʱ����N2��H2��NH3�����ʵ����ֱ�Ϊ2mol��1mol��2mol�����ʱ��ӦV(N2)�� V(N2)���������������������ȷ������
��500�桢50MPaʱ�����ݻ�Ϊ2L�������м���1 mol N2��3 mol H2����Ӧ��ƽ�����ƽ�ⳣ��ΪK����ʱN2��ת����Ϊx����K��x�Ĺ�ϵ����K�� ��
����������ͬ�����и�����1 molN2��3molH2����ijһ��ͬ�����·�Ӧ���ﵽƽ�⣬�������������ʱ��仯��������ͼ������˵����ȷ���� ������ţ���
A��ͼ������Dz�ͬѹǿ�Է�Ӧ��Ӱ�죬��P2��P1
B��ͼ������Dz�ͬѹǿ�Է�Ӧ��Ӱ�죬��P1��P2
C��ͼ������Dz�ͬ�¶ȶԷ�Ӧ��Ӱ�죬��T1��T2
D��ͼ�������ͬ��ͬѹ�£��������ܣ�1��2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���㽭ʡ���ݵ�����У�����߶��ڶ�ѧ�����л�ѧ�Ծ� ���ͣ������
��12�֣��ϳɰ����������Ĵ����������˹��̵���;�����Ի�ѧ��ҵ����Ҳ�������ش�Ӱ�졣�ϳɰ���Ӧ�Ļ�ѧ����ʽΪ��N2(g)��3H2(g) 3NH3(g) ��H����92.2kJ/mol���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2����̼�ڸ�������ˮ������Ӧ�Ƶá��ҹ��ϳɰ���ҵĿǰ����������Ϊ������������ý���¶ȣ�400��500�棬ѹǿ��30��50MPa���ش��������⣺
3NH3(g) ��H����92.2kJ/mol���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2����̼�ڸ�������ˮ������Ӧ�Ƶá��ҹ��ϳɰ���ҵĿǰ����������Ϊ������������ý���¶ȣ�400��500�棬ѹǿ��30��50MPa���ش��������⣺
��1����ˮ����ͨ�����ȵ�̿������ˮú����
C(s) + H2O(g)  H2(g) + CO(g) ��H =" +131.3" kJ ����S =" +133.7" J/K
H2(g) + CO(g) ��H =" +131.3" kJ ����S =" +133.7" J/K
�÷�Ӧ�ڵ������ܷ��Է� ����ܻ��
��2���ϳɰ���ҵ��ԭ����ѹ����30��50MPa��ԭ���� ����ƽ���ƶ�ԭ������������������ԭ������ת����ʵ�������в���400��500��ĸ��£�ԭ��֮һ�ǿ��ǵ������Ĵ����ԣ�ԭ��֮���� ��
��3����֪��400��ʱ��N2 (g)+ 3H2(g) 2NH3(g) ��K=0.5��
2NH3(g) ��K=0.5��
����400��ʱ�� 2NH3(g) N2 (g)+ 3H2(g)��K= ������ֵ����
N2 (g)+ 3H2(g)��K= ������ֵ����
��400��ʱ����0.5L�ķ�Ӧ�����н��кϳɰ���Ӧ��һ��ʱ����N2��H2��NH3�����ʵ����ֱ�Ϊ2mol��1mol��2mol�����ʱ��ӦV(N2)�� V(N2)���������������������ȷ������
��500�桢50MPaʱ�����ݻ�Ϊ2L�������м���1 mol N2��3mol H2����Ӧ��ƽ�����ƽ�ⳣ��ΪK����ʱN2��ת����Ϊx����K��x�Ĺ�ϵ����K�� ��
����������ͬ�����и�����1 molN2��3molH2����ijһ��ͬ�����·�Ӧ���ﵽƽ�⣬�������������ʱ��仯��������ͼ������˵����ȷ���� ������ţ���
| A��ͼ������Dz�ͬѹǿ�Է�Ӧ��Ӱ�죬��P2��P1 |
| B��ͼ������Dz�ͬѹǿ�Է�Ӧ��Ӱ�죬��P1��P2 |
| C��ͼ������Dz�ͬ�¶ȶԷ�Ӧ��Ӱ�죬��T1��T2 |
| D��ͼ�������ͬ��ͬѹ�£��������ܣ�1��2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���㽭ʡ���ݵ�����У�����߶��ڶ�ѧ�����л�ѧ�Ծ� ���ͣ������
��12�֣��ϳɰ����������Ĵ����������˹��̵���;�����Ի�ѧ��ҵ����Ҳ�������ش�Ӱ�졣�ϳɰ���Ӧ�Ļ�ѧ����ʽΪ��N2(g)��3H2(g) 3NH3(g)
��H����92.2kJ/mol���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2����̼�ڸ�������ˮ������Ӧ�Ƶá��ҹ��ϳɰ���ҵĿǰ����������Ϊ������������ý���¶ȣ�400��500�棬ѹǿ��30��50MPa���ش��������⣺
3NH3(g)
��H����92.2kJ/mol���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2����̼�ڸ�������ˮ������Ӧ�Ƶá��ҹ��ϳɰ���ҵĿǰ����������Ϊ������������ý���¶ȣ�400��500�棬ѹǿ��30��50MPa���ش��������⣺
��1����ˮ����ͨ�����ȵ�̿������ˮú����
C(s) + H2O(g)  H2(g) +
CO(g) ��H = +131.3 kJ ����S = +133.7 J/K
H2(g) +
CO(g) ��H = +131.3 kJ ����S = +133.7 J/K
�÷�Ӧ�ڵ������ܷ��Է� ����ܻ��
��2���ϳɰ���ҵ��ԭ����ѹ����30��50MPa��ԭ���� ����ƽ���ƶ�ԭ������������������ԭ������ת����ʵ�������в���400��500��ĸ��£�ԭ��֮һ�ǿ��ǵ������Ĵ����ԣ�ԭ��֮���� ��
��3����֪��400��ʱ��N2 (g)+ 3H2(g)  2NH3(g) ��K=0.5��
2NH3(g) ��K=0.5��
����400��ʱ�� 2NH3(g) N2 (g)+ 3H2(g)��K=
������ֵ����
N2 (g)+ 3H2(g)��K=
������ֵ����
��400��ʱ����0.5L�ķ�Ӧ�����н��кϳɰ���Ӧ��һ��ʱ����N2��H2��NH3�����ʵ����ֱ�Ϊ2mol��1mol��2mol�����ʱ��ӦV(N2)�� V(N2)���������������������ȷ������
��500�桢50MPaʱ�����ݻ�Ϊ2L�������м���1 mol N2��3 mol H2����Ӧ��ƽ�����ƽ�ⳣ��ΪK����ʱN2��ת����Ϊx����K��x�Ĺ�ϵ����K�� ��
����������ͬ�����и�����1 molN2��3molH2����ijһ��ͬ�����·�Ӧ���ﵽƽ�⣬�������������ʱ��仯��������ͼ������˵����ȷ���� ������ţ���

A��ͼ������Dz�ͬѹǿ�Է�Ӧ��Ӱ�죬��P2��P1
B��ͼ������Dz�ͬѹǿ�Է�Ӧ��Ӱ�죬��P1��P2
C��ͼ������Dz�ͬ�¶ȶԷ�Ӧ��Ӱ�죬��T1��T2
D��ͼ�������ͬ��ͬѹ�£��������ܣ�1��2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����08������һģ����10�֣��ϳɰ����������Ĵ����������˹��̵���;�����Ի�ѧ��ҵ����Ҳ�������ش�Ӱ�졣�ϳɰ���Ӧ�Ļ�ѧ����ʽΪ��N2(g)��3H2(g)![]() 2NH3(g) ��H����92.2kJ/mol���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2���ü����ڸ�������ˮ������Ӧ�Ƶá��ҹ��ϳɰ���ҵĿǰ����������Ϊ��
2NH3(g) ��H����92.2kJ/mol���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2���ü����ڸ�������ˮ������Ӧ�Ƶá��ҹ��ϳɰ���ҵĿǰ����������Ϊ��
����������ý���¶ȣ�400��500�棬ѹǿ��30��50MPa��
�ش��������⣺
�� �ϳɰ���ҵ��ԭ����ѹ����30��50MPa��ԭ���� ����ƽ���ƶ�ԭ������������������ԭ������ת����ʵ�������в���400��500��ĸ��£�ԭ��֮һ�ǿ��ǵ������Ĵ����ԣ�ԭ��֮���� ��
�� 500�桢50MPaʱ�����ݻ�ΪVL�������м���n mol N2��3n mol H2����Ӧ��ƽ�����ƽ�ⳣ��ΪK����ʱN2��ת����Ϊx����K��x�Ĺ�ϵ����K�� ��
����������Ѱ����ʵĴ����͵缫���ϣ���N2��H2Ϊ�缫��Ӧ���HCl��NH4ClΪ�������Һ��ȡ����ȼ�ϵ�ء���д���õ�ص�������Ӧʽ ��
����֪H2(g)�� CO(g) ��CH4(g)�������ʵ�ȼ���ȷֱ��ǣ�285.8 kJ?mol��1�� ��283.0 kJ?mol��1��
��890.3 kJ?mol��1��1mol H2O(g)ת��Ϊ1mol H2O(l)ʱ�ų�44.0 kJ�����������ڸ�������ˮ������Ӧ�Ļ�ѧ����ʽΪ��CH4��g����H2O��g����CO��g����3H2��g������ô�÷�Ӧ�ķ�Ӧ�ȡ�H 0 ����>��= �� <����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�ϳɰ����������Ĵ����������˹��̵���;�����Ի�ѧ��ҵ����Ҳ�������ش�Ӱ�졣�ϳɰ���Ӧ�Ļ�ѧ����ʽΪ��N2(g)��3H2(g)![]() 3NH3(g) ��H����92.2kJ/mol���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2����̼�ڸ�������ˮ������Ӧ�Ƶá��ҹ��ϳɰ���ҵĿǰ����������Ϊ������������ý���¶ȣ�400��500�棬ѹǿ��30��50MPa���ش��������⣺
3NH3(g) ��H����92.2kJ/mol���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2����̼�ڸ�������ˮ������Ӧ�Ƶá��ҹ��ϳɰ���ҵĿǰ����������Ϊ������������ý���¶ȣ�400��500�棬ѹǿ��30��50MPa���ش��������⣺
��1����ˮ����ͨ�����ȵ�̿������ˮú����
C(s) + H2O(g) ![]() H2(g) + CO(g) ��H = +131.3 kJ ����S = +133.7 J/K
H2(g) + CO(g) ��H = +131.3 kJ ����S = +133.7 J/K
�÷�Ӧ�ڵ������ܷ��Է� ����ܻ��
��2���ϳɰ���ҵ��ԭ����ѹ����30��50MPa��ԭ���� ����ƽ���ƶ�ԭ������������������ԭ������ת����ʵ�������в���400��500��ĸ��£�ԭ��֮һ�ǿ��ǵ������Ĵ����ԣ�ԭ��֮���� ��
��3����֪��400��ʱ��N2 (g)+ 3H2(g) ![]() 2NH3(g) ��K=0.5��
2NH3(g) ��K=0.5��
����400��ʱ�� 2NH3(g)![]() N2 (g)+ 3H2(g)��K= ������ֵ����
N2 (g)+ 3H2(g)��K= ������ֵ����
��400��ʱ����0.5L�ķ�Ӧ�����н��кϳɰ���Ӧ��һ��ʱ����N2��H2��NH3�����ʵ����ֱ�Ϊ2mol��1mol��2mol�����ʱ��ӦV(N2)�� V(N2)���������������������ȷ������
��500�桢50MPaʱ�����ݻ�Ϊ2L�������м���1 mol N2��3 mol H2����Ӧ��ƽ�����ƽ�ⳣ��ΪK����ʱN2��ת����Ϊx����K��x�Ĺ�ϵ����K�� ��
����������ͬ�����и�����1 molN2��3molH2����ijһ��ͬ�����·�Ӧ���ﵽƽ�⣬�������������ʱ��仯��������ͼ������˵����ȷ���� ������ţ���
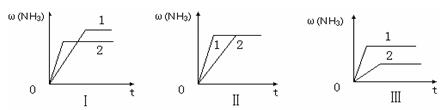
A��ͼ������Dz�ͬѹǿ�Է�Ӧ��Ӱ�죬��P2��P1
B��ͼ������Dz�ͬѹǿ�Է�Ӧ��Ӱ�죬��P1��P2
C��ͼ������Dz�ͬ�¶ȶԷ�Ӧ��Ӱ�죬��T1��T2
D��ͼ�������ͬ��ͬѹ�£��������ܣ�1��2
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com