


 ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д� ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д� ����ϰ�⽭��ϵ�д�
����ϰ�⽭��ϵ�д� ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��12 | B��7 | C��6 | D��2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��c(NH4+)��c(Cl-) | B��c(H+)=c(OH-) |
| C��c(NH4+)��c(Cl-) | D��c(OH-)��c(Cl-)��c(H+)��(NH4+) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
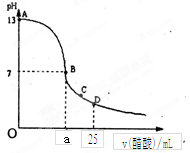
A�����Һ�У�c(H+)=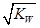 | B�����Һ��pH=7 |
| C�����Һ�У�c(B+)>c(A-) | D��a = b |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����10-8+10-10��mol/L | B��2��10-10 mol/L |
| C��1/2��10-8+10-10��mol/L | D����10-4+10-6��mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 0.05mol��
0.05mol��| A��c(Cl��)��c(NH4+)��c(H+)��c(OH��) | B��c(Cl��)��c(NH4+)��c(OH��)��c(H+) |
| C��c(Cl��)��c(H+)��c(NH4+)��c(OH��) | D��c(NH4+)��c(Cl��)��c(OH��)��c(H+) |
 ��ѡ����ţ� ��
��ѡ����ţ� �� ��<������=������ͬ�� c��NH
��<������=������ͬ�� c��NH 3��H2O���� ��Ϻ���Һ��c��NH4+����c��Cl�����Ĺ�ϵc��NH4+�� c��Cl����
3��H2O���� ��Ϻ���Һ��c��NH4+����c��Cl�����Ĺ�ϵc��NH4+�� c��Cl���� �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 H3COOH
H3COOH  .CH3COOH��NaOH�Ļ����Һ�Լ��ԣ�����Һ�и�����Ũ�ȴ�С����һ��Ϊ
.CH3COOH��NaOH�Ļ����Һ�Լ��ԣ�����Һ�и�����Ũ�ȴ�С����һ��Ϊ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ú��ʯ�͡���Ȼ����Ϊ��ʯ��Դ |
| B����ʯȼ����ȼ�չ������ܲ�����Ⱦ������SO2���к����� |
| C��ֱ��ȼ��ú���罫ú������ӹ�����ȼ��Ч���� |
| D����ʯ��Դ�ǿ�������Դ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com