
(6��)��֪��T�桢P kpaʱ���ݻ�ΪV ���ܱ������г���1molA��2molB�����ֺ��º�ѹʹ��Ӧ��ƽ�⣺A(g)+B(g)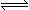 C(g)��ƽ��ʱC ���������Ϊ40�G��
C(g)��ƽ��ʱC ���������Ϊ40�G��
(1)�������¶ȡ�ѹǿ�����������²��䣬���ܱ������г���2molA��4molB����ƽ��ʱ��ϵ�ݻ�Ϊ ��C���������Ϊ ��
(2)��ȡһ���ݻ��̶�������ܱ��������Կ����¶�ΪT�棬����1molB��1molC��Ҫʹƽ��ʱC �����������Ϊ40�G������ܱ��������Ϊ ��
�� 10V/7 40% �Ƣ� 5V/7
��������
������������������¶ȡ�ѹǿ�����������²��䣬���ܱ������г���2molA��4molB�������ں��º��������µĵ�Чƽ��
A(g) + B(g) 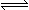 C(g)
C(g)
��ʼ���ʵ����� 1mol 2mol 0mol
ת���� xmol xmol xmol
ƽ��ʱ�����ʵ����� (1-x)mol (2-x)mol xmol
�������֪��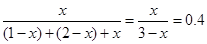
��֮�ã�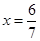 Ҳ����˵ƽ����ܵ����ʵ���Ϊ��
Ҳ����˵ƽ����ܵ����ʵ���Ϊ��
3mol�Ļ������ռ�����ΪV����ô ������ռ�����Ϊ��
������ռ�����Ϊ��
���ԣ������¶ȡ�ѹǿ�����������²��䣬���ܱ������г���2molA��4molB����ƽ��ʱ��ϵ�ݻ�Ϊ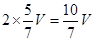 ������ƽ�ⲻ�ƶ�������������䡣
������ƽ�ⲻ�ƶ�������������䡣
�����ǵ��µ��������µĵ�Чƽ�⣬���ֻ���ǵ���ԭ����
���㣺��Чƽ����й�֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


| Cl2 |
| ���� |
| NaOH |
| ˮ���� |
| K2Cr2O7��H+ |
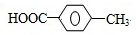



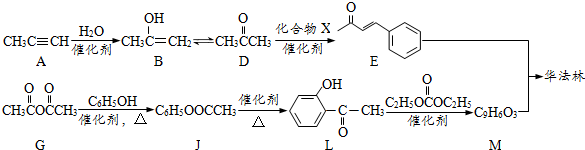
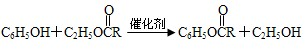 �͢�
�͢� ��M�Ľṹ��ʽΪ
��M�Ľṹ��ʽΪ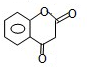

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| c2(C) |
| c(A)��c3(B) |
| c2(C) |
| c(A)��c3(B) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
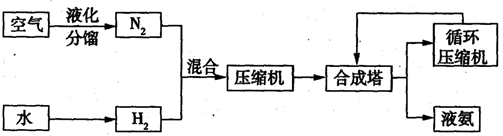
| �������� | ���� | ���� | ���� | ���� | ��� | ��� | ��� |
| �е�/�� | -196 | -183 | -269 | -264 | -186 | -153 | -108 |
| ԭ�� | ��Ȼ�� | ���� | ú |
| ���Ͷ�ʷ��� | 1.0 | 1.5 | 2.0 |
| ��������/J?t-1 | 28��109 | 38��109 | 48��109 |
| ||
| ���� |
| ||
| ���� |
| NH3����% ѹǿ/MPa �¶�/�� |
0.1 | 10 | 20 | 30 | 60 | 100 |
| 200 | 15.3 | 81.5 | 86.4 | 89.9 | 95.4 | 98.8 |
| 300 | 2.2 | 52.0 | 64.2 | 71.0 | 84.2 | 92.6 |
| 400 | 0.4 | 25.1 | 38.2 | 47.0 | 65.2 | 79.8 |
| 500 | 0.1 | 10.6 | 19.1 | 26.4 | 42.2 | 57.5 |
| 600 | 0.05 | 4.5 | 9.1 | 13.8 | 23.1 | 31.4 |
 2NH3�ġ�H
2NH3�ġ�H| c2(NH3) |
| c(N2)?c3(H2) |
| c2(NH3) |
| c(N2)?c3(H2) |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com