
���� ����c�����⣩=$\frac{c����ע����V������}{V�����⣩}$��������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
��� �⣺��1���ζ�ǰ���ζ��ܼ�״������ݣ��ζ���������ʧ�����V������ƫ����c�����⣩=$\frac{c����ע����V������}{V�����⣩}$��������֪c�����⣩ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
��2���ζ���������ˮϴ�Ӻ�װ�����Һ���еζ�����Һ��ϡ�ͣ�Ũ�ȼ�С�����V������ƫ����c�����⣩=$\frac{c����ע����V������}{V�����⣩}$��������֪c�����⣩ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
��3���ζ�ǰ����ƿ������ˮϴ�Ӻ�δ�ô��������������Һ��ϴ�����ζ���V��������Ӱ�죬����c�����⣩=$\frac{c����ע����V������}{V�����⣩}$��������֪c�����⣩���䣻
�ʴ�Ϊ����Ӱ�죻
��4�����õζ�����ȡ����Һ����������ˮϴ�ӵζ��ܣ�����ϴ��Һһ��ע����ƿ�У�����Һ�����ʵ���ƫ�࣬���V������ƫ����c�����⣩=$\frac{c����ע����V������}{V�����⣩}$��������֪c�����⣩ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
��5���ζ������У���ƿҡ�����ң�������ЩҺ�ηɽ�����������Һ�����ʵ���ƫ�٣����V������ƫС������c�����⣩=$\frac{c����ע����V������}{V�����⣩}$��������֪c�����⣩ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
���� ������Ҫ�������к͵ζ�������������������c�����⣩=$\frac{c����ע����V������}{V�����⣩}$�����ǽ��Ĺؼ�����Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���淴Ӧ�ﵽ��ѧƽ��״̬ʱ�������淴Ӧ���ʾ������� | |
| B�� | ����Ӧ����������Ũ�����ʱ�����淴Ӧһ���Ѵﵽ��ѧƽ�� | |
| C�� | ��ѧ��Ӧ���Ⱦ����˷�Ӧ���ڸ������µ����ת���� | |
| D�� | ��п����Ʒ�Ʋ����������Ʒ������ǰ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1.5mol����Fe����������������ȫȼ�գ�ʧȥ4NA������ | |
| B�� | ��25g��������Ϊ60%������ˮ��Һ�У�������ԭ����ΪNA | |
| C�� | 7.8��gNa2S��Na2O2���Ļ�����У����е�������������0.15NA | |
| D�� | ��5��107Pa��500��C������ý�����£�1molN2��3molH2�����ɰ���������Ϊ2NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
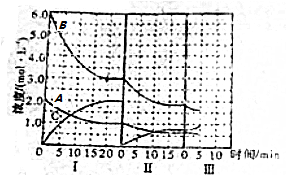
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2Na2O2+2H2O�T4NaOH+O2�� | B�� | Mg3Cl2+2NaOH�TMg��OH��2��+2NaCl | ||
| C�� | 2NaOH+Cl2�TNaOCl+H2O | D�� | NH4Cl+NaO�TNaCl+NH3��+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | S��g��+O2��g��=SO2��g���ų���������297.23kJ | |
| B�� | S��g��+O2��g��=SO2��g���ų�����С��297.23kJ | |
| C�� | ��H=-297.23kJ•mol-1��ʾ����S��ȼ���� | |
| D�� | �γ�1molSO2��ѧ���ͷŵ����������ڶ���1molS��s����1molO2��g����ѧ�����յ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com