
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
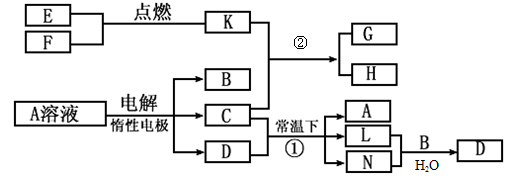
(8��)��֪AΪ��ɫ��Һ��B��C��I��KΪ���ʣ������Ϊ���������B��L��I��K������Ϊ���壬��ֻ��IΪ��ɫ���塣GΪ��ɫ���壬F����ɫ��Ӧ����ɫ�겣��Ƭ����ɫ��������ת����ϵ��ͼ��
�ش��������⣺
��1��F�ĵ���ʽ��____________��
��2��д����Ӧ�ٵĻ�ѧ��Ӧ����ʽ�� ��
��3���ö��Ե缫�������A��Һ��һ��ʱ�����Ҫʹ��Һ�ָ������ǰ��״̬���������Һ�м�������___________��__________��
��4����֪B��H��C2H5OH�ܹ����ȼ�ϵ�أ�д����ȼ�ϵ�صĸ����缫��Ӧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������һ�и������ڵ������¿� ���ͣ������
(14��)��֪AΪ��ɫ��Һ��B��C��I��KΪ���ʣ������Ϊ�����B��L��K ������Ϊ��ɫ��ζ���壬IΪ��ɫ�д̼�����ζ���塣GΪ��ɫ���壬F����ɫ��Ӧ����ɫ(���ܲ����۲�)����Ӧ���У�����F��P�����ʵ���֮��Ϊ1��1��������ת����ϵ����ͼ��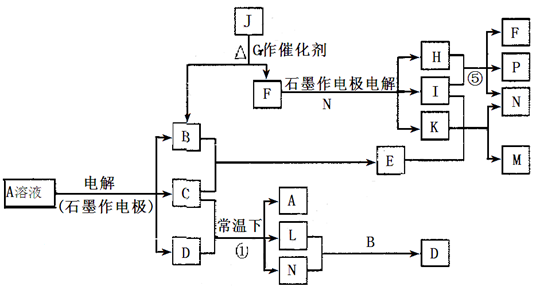
�ش��������⣺
��1��P�ĵ���ʽ��_________��I����Ԫ����Ԫ�����ڱ��е�λ����________________��
��2��д����Ӧ�ٵ����ӷ���ʽ�� ______________________________________________��
��3��M��ˮ��Һ��___________������ԡ������ԡ������ԡ����������ӷ���ʽ˵��ԭ��______________________________________________________________
��4���ö��Ե缫���400.00mL A��Һ��һ��ʱ�������ҺpH��1����ʱ��Ҫ����Һ�м���___________��������Ϊ______g������ʹ��Һ�ָ������ǰ��״̬����������Һ����仯����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��2011ѧ�����ʡ������ѧ�߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
(8��) ��֪AΪ��ɫ��Һ��B��C��I��KΪ���ʣ������Ϊ���������B��L��I��K ������Ϊ���壬��ֻ��IΪ��ɫ���塣GΪ��ɫ���壬F����ɫ��Ӧ����ɫ�겣��Ƭ����ɫ��������ת����ϵ��ͼ��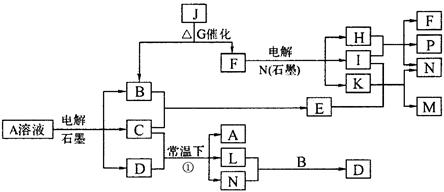
�ش��������⣺
��1��F�ĵ���ʽ��____________��
��2��д����Ӧ�ٵĻ�ѧ��Ӧ����ʽ�� ��
��3���ö��Ե缫�������A��Һ��һ��ʱ�����Ҫʹ��Һ�ָ������ǰ��״̬���������Һ�м�������___________��__________��
��4����֪B��H��C2H5OH�ܹ����ȼ�ϵ�أ�д����ȼ�ϵ�صĸ����缫��Ӧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
(8��) ��֪AΪ��ɫ��Һ��B��C��I��KΪ���ʣ������Ϊ���������B��L��I��K ������Ϊ���壬��ֻ��IΪ��ɫ���塣GΪ��ɫ���壬F����ɫ��Ӧ����ɫ�겣��Ƭ����ɫ��������ת����ϵ��ͼ��
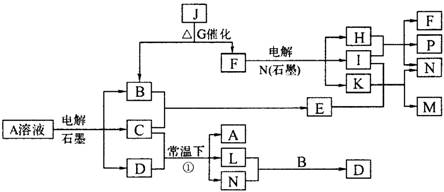
�ش��������⣺
��1��F�ĵ���ʽ��____________��
��2��д����Ӧ�ٵĻ�ѧ��Ӧ����ʽ�� ��
��3���ö��Ե缫�������A��Һ��һ��ʱ�����Ҫʹ��Һ�ָ������ǰ��״̬���������Һ�м�������___________��__________��
��4����֪B��H��C2H5OH�ܹ����ȼ�ϵ�أ�д����ȼ�ϵ�صĸ����缫��Ӧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪AΪ��ɫ��Һ��B��C��I��KΪ���ʣ������Ϊ���������B��L��I��K ������Ϊ���壬��ֻ��IΪ��ɫ���塣GΪ��ɫ���壬F����ɫ��Ӧ����ɫ�겣��Ƭ����ɫ��������ת����ϵ��ͼ��
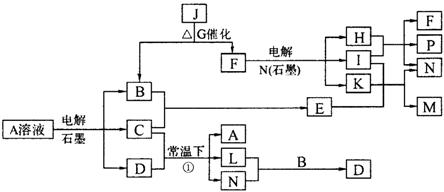
�ش��������⣺
��1��F�ĵ���ʽ��____________��
��2��д����Ӧ�ٵĻ�ѧ��Ӧ����ʽ�� ��
��3���ö��Ե缫�������A��Һ��һ��ʱ�����Ҫʹ��Һ�ָ������ǰ��״̬���������Һ�м�������___________��__________��
��4����֪B��H��C2H5OH�ܹ����ȼ�ϵ�أ�д����ȼ�ϵ�صĸ����缫��Ӧ����ʽ�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com