
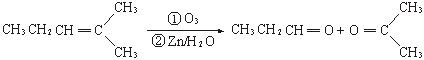
��.��֪��ȩ��ȼ����Ϊ1815 kJ/mol����ͪ��ȼ����Ϊ1789 kJ/mol����д����ȩȼ�յ��Ȼ�ѧ����ʽ ��
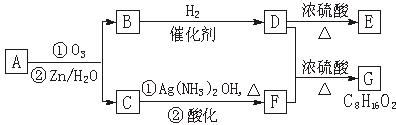
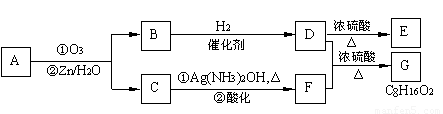
��.������Ӧ�������ƶ�ϩ���Ľṹ��һ����״��ϩ��Aͨ������������п��ˮ�����õ�B��C��������B��̼69.8%������11.6%��B��������Ӧ������������D��D��Ũ��������¼��ȣ��ɵõ���ʹ��ˮ��ɫ��ֻ��һ�ֽṹ������E����Ӧͼʾ���£�
�ش��������⣺
��1��B����Է��������� ��C��F�ķ�Ӧ����Ϊ ��D�к��й����ŵ����� ��
��2��D+F��G�Ļ�ѧ����ʽ�� ��
��3��A�Ľṹ��ʽΪ ��
��4��������A��ij��ͬ���칹��ͨ������������п��ˮ����ֻ�õ�һ�ֲ�����ϸ��������칹��Ľṹ��ʽ�� �֡�
 ����ѧУ�ֲ����ܲ�ϵ�д�
����ѧУ�ֲ����ܲ�ϵ�д� �ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
�ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2011���㽭ʡ�߶���12�¼�⻯ѧ�Ծ� ���ͣ������
��10�֣�ϩ��ͨ������������п��ˮ�����õ�ȩ��ͪ�����磺
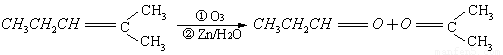
������Ӧ�������ƶ�ϩ���Ľṹ��һ����״��ϩ��Aͨ������������п��ˮ�����õ�B��C��������B��̼69.8%������11.6%��B��������Ӧ������������D��D��Ũ��������¼��ȣ��ɵõ���ʹ��ˮ��ɫ��ֻ��һ�ֽṹ������E����Ӧͼʾ���£�
��1��A�Ľṹ��ʽΪ ��
��2��D �� E�ķ�Ӧ����Ϊ ��B�к��й����ŵ����� ��G��������������̼ԭ�ӣ� ��D���ʵĺ˴Ź�����ͼ���м����壿 ��
��3��д����ѧ����ʽ��
C��F ��
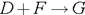 ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

ijϩ��A�ķ���ʽΪC5H6����������ת����
��1��Aͨ������������п��ˮ�����õ�B��C��B��C��Ϊͬϵ���B��C��1��̼ԭ�ӡ�
��2��B��C���ܷ���������Ӧ����B��������H2�ڴ��������¿ɵõ�D����C��������Һ��Ӧ���ữ�ɵõ�E��
��3��D��E��һ�������·�Ӧ�ɵõ����ֲ�ͬ�л�����F��G��H������F�ķ���ʽΪ��C5H6O4��n��G�ķ���ʽΪC5H6O4��H�ķ���ʽΪC5H8O5��
��
��1��A�Ľṹ��ʽΪ__________��F������Ϊ__________��
��2��D+E![]() F�Ļ�ѧ����ʽ��____________________________��
F�Ļ�ѧ����ʽ��____________________________��
C��������Һ��Ӧ�Ļ�ѧ����ʽ��______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�����߿����� ���ͣ��ƶ���

 ����ͪ��ȼ����Ϊ1789
����ͪ��ȼ����Ϊ1789 ����д����ȩȼ�յ��Ȼ�ѧ����ʽ_____________ ��
����д����ȩȼ�յ��Ȼ�ѧ����ʽ_____________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��04����������19�֣�ϩ��ͨ������������п��ˮ�����õ�ȩ��ͪ�����磺
![]()
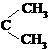
![]()
![]()
![]()
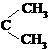
I. ��֪��ȩ��ȼ����Ϊ![]() ����ͪ��ȼ����Ϊ
����ͪ��ȼ����Ϊ![]() ����д����ȩȼ�յ��Ȼ�ѧ����ʽ ��
����д����ȩȼ�յ��Ȼ�ѧ����ʽ ��
II. ������Ӧ�������ƶ�ϩ���Ľṹ��һ����״��ϩ��Aͨ������������п��ˮ�����õ�B��C��������B��̼69.8%������11.6%��B��������Ӧ������������D��D��Ũ��������¼��ȣ��ɵõ���ʹ��ˮ��ɫ��ֻ��һ�ֽṹ������E����Ӧͼʾ���£�
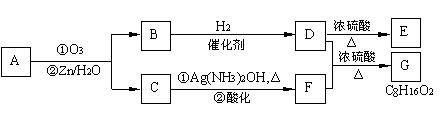
�ش��������⣺
��1��B����Է��������� ��C![]() F�ķ�Ӧ����Ϊ ��D�к��й����ŵ����� ��
F�ķ�Ӧ����Ϊ ��D�к��й����ŵ����� ��
��2��![]() �Ļ�ѧ����ʽ�ǣ�
�Ļ�ѧ����ʽ�ǣ�
��
��3��A�Ľṹ��ʽΪ ��
��4��������A��ij��ͬ���칹��ͨ������������п��ˮ����ֻ�õ�һ�ֲ�����ϸ��������칹��Ľṹ��ʽ�� �֡�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com