
��11�֣������������������Լ�����Ӱ���ȣ����Ʊ��������£�
��1������(NH4)2Fe(SO4)2 6H2O��Һʱ���������ϡ���ᣬĿ����________________________________________________________��
6H2O��Һʱ���������ϡ���ᣬĿ����________________________________________________________��
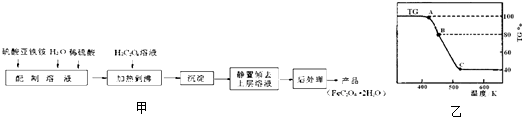
��2�����ƵõIJ�Ʒ����������н������ط����������ͼ10��TG%��ʾ������������ռԭ��Ʒ�������İٷ�������
��A B������Ӧ�Ļ�ѧ����ʽΪ
B������Ӧ�Ļ�ѧ����ʽΪ
__________________________________________��
C��ʱ������Ļ�ѧʽΪ______________��
�����о�ѧ����ʵ�������������ɫ�����
H2�����ղ�����Ҳ�����Ĵ����������ɣ�_____________________________
���������һ������ʽ����������ʵ��______________________________ ��
�� ��ȡ�������146����ˮ���FeC2O41.44g����ij��յ��ܱ������У��ٳ���0.04molCO��������1100�棬����FeO(s)+CO(g) Fe(s)+CO2(g)��Ӧƽ�ⳣ��K=0.4����÷�Ӧ��ƽ��ʱ��FeO��ת����Ϊ���٣�____________________________��
Fe(s)+CO2(g)��Ӧƽ�ⳣ��K=0.4����÷�Ӧ��ƽ��ʱ��FeO��ת����Ϊ���٣�____________________________��
 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(14��) �����������������Լ�����Ӱ���ȣ����Ʊ��������£�
������(NH4)2Fe(SO4)26H2O��Һʱ���������ϡ���ᣬĿ���� ��
�ƽ��ƵõIJ�Ʒ����������н������ط������������ͼ��TG%��ʾ������������ռԭ��Ʒ�������İٷ�������
����C��ʱ������Ļ�ѧʽΪ ��
�����о�ѧ����ʵ�������������ɫ�����H2�����ղ� ����Ҳ�����Ĵ����������ɣ����������һ������ʽ����������ʵ�� ��
����ȡ�������146����ˮ���FeC2O41.44g����ij��յ��ܱ������У��ٳ���0.04molCO��������1100�棬����FeO(s)+CO(g)Fe(s)+CO2(g)��Ӧƽ�ⳣ��K=0.4����÷�Ӧ��ƽ��ʱ��FeO��ת����Ϊ���٣� ��
��3������þ�ڹ�������������Ҫ���ã�����MgCl2Ϊԭ�ϻ�ȡ���¶Ⱥ�ѹǿP(HCl)g��MgCl2��6H2O�����ȷֽ�����Ӱ����ͼ��ʾ�������ͼ��ش��������⣺
��д��P(HCl)g = 0.25MPa���¶ȴ�300�����ߵ�550��ʱ��Ӧ�Ļ�ѧ����ʽ ��
��ʵ�������У���MgCl2��6H2O������ȵ�600��Ĺ����м����ò�����ˮMgCl2����ԭ���� ����Ҫ�õ���ˮMgCl2���ȡ�Ĵ�ʩ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ������ѧ����4��˫����ϰ��ѧ�Ծ����������� ���ͣ������
(14��) �����������������Լ�����Ӱ���ȣ����Ʊ��������£�
������(NH4)2Fe(SO4)2 6H2O��Һʱ���������ϡ���ᣬĿ���� ��
6H2O��Һʱ���������ϡ���ᣬĿ���� ��
�ƽ��ƵõIJ�Ʒ����������н������ط������������ͼ��TG%��ʾ������������ռԭ��Ʒ�������İٷ�������
����C��ʱ������Ļ�ѧʽΪ ��
�����о�ѧ����ʵ�������������ɫ�����H2�����ղ�����Ҳ�����Ĵ����������ɣ����������һ������ʽ����������ʵ�� ��
����ȡ�������146����ˮ���FeC2O41.44g����ij��յ��ܱ������У��ٳ���0.04molCO��������1100�棬����FeO(s)+CO(g) Fe(s)+CO2(g)��Ӧƽ�ⳣ��K=0.4����÷�Ӧ��ƽ��ʱ��FeO��ת����Ϊ���٣� ��
Fe(s)+CO2(g)��Ӧƽ�ⳣ��K=0.4����÷�Ӧ��ƽ��ʱ��FeO��ת����Ϊ���٣� ��
��3������þ�ڹ�������������Ҫ���ã�����MgCl2Ϊԭ�ϻ�ȡ���¶Ⱥ�ѹǿP(HCl)g��MgCl2��6H2O�����ȷֽ�����Ӱ����ͼ��ʾ�������ͼ��ش��������⣺
��д��P(HCl)g = 0.25MPa���¶ȴ�300�����ߵ�550��ʱ��Ӧ�Ļ�ѧ����ʽ ��
��ʵ�������У���MgCl2��6H2O������ȵ�600��Ĺ����м����ò�����ˮMgCl2����ԭ���� ����Ҫ�õ���ˮMgCl2���ȡ�Ĵ�ʩ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ������ѧ�ڿ�ѧ������⻯ѧ�Ծ� ���ͣ������
(14��) �����������������Լ�����Ӱ���ȣ����Ʊ��������£�

������(NH4)2Fe(SO4)2 6H2O��Һʱ���������ϡ���ᣬĿ����
��
6H2O��Һʱ���������ϡ���ᣬĿ����
��
�ƽ��ƵõIJ�Ʒ����������н������ط������������ͼ��TG%��ʾ������������ռԭ��Ʒ�������İٷ�������

����C��ʱ������Ļ�ѧʽΪ ��
�����о�ѧ����ʵ�������������ɫ�����H2�����ղ� ����Ҳ�����Ĵ����������ɣ����������һ������ʽ����������ʵ�� ��
����ȡ�������146����ˮ���FeC2O41.44g����ij��յ��ܱ������У��ٳ���0.04molCO��������1100�棬����FeO(s)+CO(g) Fe(s)+CO2(g)��Ӧƽ�ⳣ��K=0.4����÷�Ӧ��ƽ��ʱ��FeO��ת����Ϊ���٣� ��
Fe(s)+CO2(g)��Ӧƽ�ⳣ��K=0.4����÷�Ӧ��ƽ��ʱ��FeO��ת����Ϊ���٣� ��
��3������þ�ڹ�������������Ҫ���ã�����MgCl2Ϊԭ�ϻ�ȡ���¶Ⱥ�ѹǿP(HCl)g��MgCl2��6H2O�����ȷֽ�����Ӱ����ͼ��ʾ�������ͼ��ش��������⣺

��д��P(HCl)g = 0.25MPa���¶ȴ�300�����ߵ�550��ʱ��Ӧ�Ļ�ѧ����ʽ ��
��ʵ�������У���MgCl2��6H2O������ȵ�600��Ĺ����м����ò�����ˮMgCl2����ԭ���� ����Ҫ�õ���ˮMgCl2���ȡ�Ĵ�ʩ�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com