
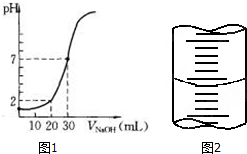
 ��һ�����ʵ���Ũ�ȵ�NaOH��Һ�ζ�10.00mL��֪Ũ�ȵ����ᣬ�ζ������ͼ1��ʾ���ش��������⣺
��һ�����ʵ���Ũ�ȵ�NaOH��Һ�ζ�10.00mL��֪Ũ�ȵ����ᣬ�ζ������ͼ1��ʾ���ش��������⣺���� ��1������c�����⣩=$\frac{c��������V������}{V�����⣩}$��������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
��2�����ݴ���Һ�м����̪����ҺΪ��ɫ����Ӧ��ȫ���������ƹ�������Һ��ɺ�ɫ�жϴﵽ�յ�����
��3������ͼ��֪����V��NaOH��=30mLʱ�������Һ�����ԣ�˵��n��HCl��=n��NaOH��������������10mL���������Ƶ������30mL����c��HCl��=3c��NaOH����������������Һ���Ϊ20mLʱ�������Һ��pH=2�����c��H+��=$\frac{c��HCl����V��HCl��-c��NaOH����V��NaOH��}{V��HCl��+V��NaOH��}$������Ũ�ȣ�
��4�����ݵζ��ܵĽṹ�����
��� �⣺��1�����ζ�ǰ���ӵζ��ܶ������ζ���ƽ�ӿ̶ȶ��������V�����⣩ƫ����c�����⣩=$\frac{c��������V������}{V�����⣩}$��֪���ⶨc�����⣩ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
��2�������м����̪����Һ��ʾ��ɫ�����ᷴӦ��ȫ��������������Һ����Һ��ʾ��ɫ�����Եζ��յ�Ϊ�����һ�ε���ʱ����ƿ����Һǡ�ó��ֺ�ɫ���Ұ�����ڲ���ɫ��
�ʴ�Ϊ�����һ�ε���ʱ����ƿ����Һǡ�ó��ֺ�ɫ���Ұ�����ڲ���ɫ��
��3������ͼ��֪����V��NaOH��=30mLʱ�������Һ�����ԣ�˵��n��HCl��=n��NaOH��������������10mL���������Ƶ������30mL����c��HCl��=3c��NaOH����������������Һ���Ϊ20mLʱ�������Һ��pH=2��c��H+��=$\frac{c��HCl����V��HCl��-c��NaOH����V��NaOH��}{V��HCl��+V��NaOH��}$=$\frac{3c��NaOH����0.01L-c��NaOH����0.02L}{0.01L+0.02L}$=0.01mol/L������c��NaOH��=0.03mol/L��
�ʴ�Ϊ��0.03��
��4�����ڵζ����¶���һ��û�п̶ȣ��ζ�����Һ������һ�����ڣ�50-a��mL��
�ʴ�Ϊ��D��
���� ���⿼��������к͵ζ�����������������Һ��������Ũ�ȵļ��㣬������Һ���ȷ�����Ũ�ȹ�ϵ������ɣ�ע�Ȿ�����ô���Һ�α�Һ���Ѷ��еȣ�


 �ǻ�С��ϰϵ�д�
�ǻ�С��ϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| �� | C | D | F | |||||
| �� | B | E | G | R | ||||
| �� | A | H |
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
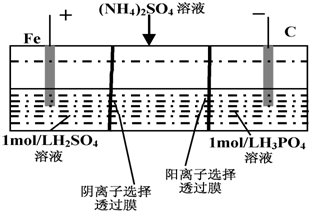
| A�� | ��������Һ����ɫ����ػ�ɫ | |
| B�� | �����ĵ缫��ӦʽΪ��4OH--4e-=2H2O+O2�� | |
| C�� | ���һ��ʱ�����������Һ�е����Լ��� | |
| D�� | ���һ��ʱ�����������Һ�е�����һ���ǣ�NH4��3PO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 11.2 L�����������ķ�����Ϊ0.5NA | |
| B�� | 28 g��ϩ�������õ��Ӷ���ĿΪ6NA | |
| C�� | �ڱ�״����11.2 L���ȼ�������������Ϊ0.5NA | |
| D�� | ������ϩ����Ȳ�Ļ�����干14 g����ԭ����Ϊ3NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | pH=a�İ�ˮ��Һ��ϡ��10������pH=b����a=b+1 | |
| B�� | Na2C2O4��Һ��c��OH-��=c��H+��+c��HC2O4-��+2c��H2C2O4�� | |
| C�� | Na2CO3��Һ��c��Na+��=2c��CO32-��+c��HCO3-�� | |
| D�� | CH3COONa��CaCl2�����Һ��c��Na+��+c��Ca2+��+c��H+��=c��CH3COO-��+c��OH?��+c��Cl-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaOH��Һ��������Ca��HCO3��2��Һ��Ӧ��2HCO3-+2OH-+Ca2+�TCaCO3��+2H2O | |
| B�� | ��ͭΪ�缫��ⱥ��ʳ��ˮ��2Cl-+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$Cl2��+H2��+2OH- | |
| C�� | FeBr2��Һ��ͨ�����Cl2��2Fe2++2Br-+2Cl2�T2Fe3++Br2+4Cl- | |
| D�� | Ba��OH��2��Һ�еμ�NaHSO4��Һ�����ԣ�Ba2++2OH-+2H++SO42-�TBaSO4��+2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����[KAl��SO4��2•12H2O]��ˮ�����γ�Al��OH��3���壬��������ˮ�� | |
| B�� | ���ʯ����Ȼ����Ӳ���������ʣ�������������������Ӧ | |
| C�� | ������Fe��Al��ŨHNO3�����ۻ�����Fe��Al������ŨHNO3������Ӧ | |
| D�� | ��SO2ͨ��Ʒ����Һ����Һ��ɫ����Ȼָ�ԭɫ����SO2ͨ����ˮ����ˮ��ɫ�����Ҳ�ָܻ�ԭɫ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com