
µ™‘™ЋЎµƒїѓЇѕќп÷÷јаЈ±ґа£ђ–‘÷ “≤Єч≤їѕаЌђ°£
£®1£©NO2”–љѕ«њµƒ—хїѓ–‘£ђƒ№љЂSO2—хїѓ…ъ≥…SO3£ђ±Њ…н±їїє‘≠ќ™NO£ђ“—÷™ѕ¬Ѕ–ЅљЈі”¶єэ≥ћ÷–ƒ№Ѕњ±дїѓ»зЌЉЋщ Њ£Ї

‘тNO2—хїѓSO2µƒ»»їѓ—ІЈљ≥ћ љќ™_________________________________°£
£®2£©‘Џ2L√№±’»Ё∆ч÷–Ј≈»л1mol∞±∆ш£ђ‘Џ“їґ®ќ¬ґ»љш––»зѕ¬Јі”¶£Ї
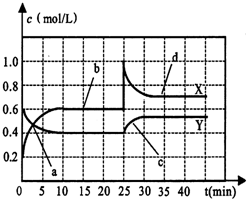
2NH3(g) N2£®g£©+3H2£®g£©£ђЈі”¶ ±Љд(t)”л»Ё∆чƒЏ∆шће„№—є«њ(p)µƒ эЊЁЉыѕ¬±н
N2£®g£©+3H2£®g£©£ђЈі”¶ ±Љд(t)”л»Ё∆чƒЏ∆шће„№—є«њ(p)µƒ эЊЁЉыѕ¬±н
±Љдt/min | 0 | 1 | 2 | 3 | 4 | 5 |
„№—є«њp 100 kPa | 5 | 5.6 | 6.4 | 6.8 | 7 | 7 |
‘т∆љЇв ±∞±∆шµƒ„™їѓ¬ ќ™___________°£
£®3£©л¬£®N2H4£©”÷≥∆Ѕ™∞±£ђ «“ї÷÷њ…»Љ–‘µƒ“Їће£ђњ…”√„чїрЉэ»ЉЅѕ°£‘Џњ’∆ш÷–Ќк»Ђ»Љ…’…ъ≥…µ™∆ш£ђµ±Јі”¶„™“∆0.2molµз„” ±£ђ…ъ≥…∆шће‘Џ±к„Љ„іњцѕ¬µƒћеїэќ™______________°£Ѕ™∞±»№”ЏЋЃњ…“‘ЈҐ…ъ”л∞±ЋЃјаЋ∆µƒµзјл£ђ ‘–і≥цЅ™∞±‘ЏЋЃ»№“Ї÷–µƒµзјлЈљ≥ћ љ£Ї
__________________£®–і“ї≤љЉіњ…£©°£
£®4£©NH4+‘Џ»№“Ї÷–ƒ№ЈҐ…ъЋЃљвЈі”¶°£‘Џ25°ж ±£ђ0.1mol/L¬»їѓпІ»№“Ї”…ЋЃµзјл≥цµƒ«вјл„”≈®ґ»ќ™1°Ѕ10-5 mol/L£ђ‘т‘ЏЄ√ќ¬ґ»ѕ¬іЋ»№“Ї÷–∞±ЋЃµƒµзјл∆љЇв≥£ эKb£®NH3°§H2O£©=__________________°£
£®1£©NO2£®g£©+SO2£®g£©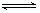 SO3£®g£©+NO£®g£© ¶§H=£≠41.8kJ/mol£®2Ј÷£©
SO3£®g£©+NO£®g£© ¶§H=£≠41.8kJ/mol£®2Ј÷£©
£®2£©40%£®2Ј÷£©
£®3£© 1.12L£®2Ј÷£©£їN2H4£ЂH2O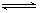 N2H5£Ђ£ЂOH£≠ £®2Ј÷£©
N2H5£Ђ£ЂOH£≠ £®2Ј÷£©
£®4£© Kb£®NH3°§H2O£©£љ1°Ѕ10-5mol/L£®2Ј÷£©
°Њљвќц°њ
‘ћвЈ÷ќц£Ї£®1£©ЄщЊЁЌЉ1њ…µ√£Ї2SO2(g)+O2(g) 2SO3(g)? ¶§H=£≠196.6kJ?mol?1£ђЄщЊЁЌЉ2њ…µ√£Ї2NO(g)+O2(g)=2NO2(g)? ¶§H=£≠113.0kJ?mol?1£ђЄщЊЁЄ«Ћєґ®¬…њ…µ√NO2£®g£©+SO2£®g£©
2SO3(g)? ¶§H=£≠196.6kJ?mol?1£ђЄщЊЁЌЉ2њ…µ√£Ї2NO(g)+O2(g)=2NO2(g)? ¶§H=£≠113.0kJ?mol?1£ђЄщЊЁЄ«Ћєґ®¬…њ…µ√NO2£®g£©+SO2£®g£©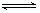 SO3£®g£©+NO£®g£© ¶§H=1/2¶§H1°™1/2¶§H2=£≠41.8kJ?mol?1
SO3£®g£©+NO£®g£© ¶§H=1/2¶§H1°™1/2¶§H2=£≠41.8kJ?mol?1
£®2£©∆шћеµƒ—є«њ÷Ѓ±»µ»”Џќп÷ µƒЅњ÷Ѓ±»£ђЄщЊЁ»эґќ љљш––Љ∆Ћг£Ї…и∞±∆шµƒ„™їѓ¬ ќ™x
???????????????????? 2NH3(g) N2£®g£©+3H2£®g£©
N2£®g£©+3H2£®g£©
∆р Љќп÷ µƒЅњ£®mol£©?? 1???????? 0??????? 0
„™їѓќп÷ µƒЅњ£®mol£©?? x???????? x/2????? 3/2x
∆љЇвќп÷ µƒЅњ£®mol£©? 1-x??????? x/2?????? 3/2x
1£Ї£®1+x£©=5:7£ђљвµ√x=40%
£®3£©N2H4„™їѓќ™N2£ђN‘™ЋЎ”… -2Љџ…эЄя÷Ѕ0Љџ£ђЋщ“‘µз„”„™“∆ќ™£ЇN2H4 ~ N2 ~ 4e?£ђn£®N2£©=1/4n£®e?£©=0.05mol£ђє ‘Џ±к„Љ„іњцѕ¬µƒћеїэќ™1.12L£їЅ™∞±»№”ЏЋЃњ…“‘ЈҐ…ъ”л∞±ЋЃјаЋ∆µƒµзјл£ђN2H4”лH+–ќ≥…≈дќїЉь£ђЋщ“‘µзјлЈљ≥ћ љќ™£ЇN2H4£ЂH2O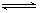 N2H5£Ђ£ЂOH£≠
N2H5£Ђ£ЂOH£≠
£®4£©c(OH?)=Kw/c(H+)=10-9mol?L?1£ђc(NH3?H2O)=c(H+)=10-5mol?L?1£ђЋщ“‘Kb£®NH3°§H2O£©=c(NH4+)?c(OH?)/c(NH3?H2O)=0.1mol/L°Ѕ10-9mol?L?1/10-5mol?L?1=1°Ѕ10-5mol/L
њЉµг£Ї±ЊћвњЉ≤й»»їѓ—ІЈљ≥ћ љµƒ й–і°Ґїѓ—І∆љЇвЇЌ∆љЇв≥£ эµƒЉ∆Ћг°ҐµзјлЈљ≥ћ љµƒ й–і°£


 њмј÷5Љ”2љрЊнѕµЅ–ір∞Є
њмј÷5Љ”2љрЊнѕµЅ–ір∞Є
| ƒкЉґ | Єя÷–њќ≥ћ | ƒкЉґ | ≥х÷–њќ≥ћ |
| Єя“ї | Єя“ї√вЈ—њќ≥ћЌ∆Љц£° | ≥х“ї | ≥х“ї√вЈ—њќ≥ћЌ∆Љц£° |
| Єяґю | Єяґю√вЈ—њќ≥ћЌ∆Љц£° | ≥хґю | ≥хґю√вЈ—њќ≥ћЌ∆Љц£° |
| Єя»э | Єя»э√вЈ—њќ≥ћЌ∆Љц£° | ≥х»э | ≥х»э√вЈ—њќ≥ћЌ∆Љц£° |
њ∆ƒњ£ЇЄя÷–їѓ—І јі‘і£Ї ћв–Ќ£Ї
 ґћ÷№∆Џ÷ч„е‘™ЋЎA°ҐB°ҐC°ҐD°ҐEµƒ‘≠„”–т э“јіќ‘ціу£ђЋь√«‘≠„”ЇЋЌвµƒµз„”≤г э÷ЃЇЌќ™10£їB‘™ЋЎµƒїѓЇѕќп÷÷јаЈ±ґа£ђ эƒњ≈”іу£ЃC°ҐDЅљ÷÷‘™ЋЎ–ќ≥…µƒµ•÷ «њ’∆ш÷–ЇђЅњ„оґаµƒќп÷ £їD°ҐEЅљ‘™ЋЎњ…“‘…ъ≥…Ѕљ÷÷≤їЌђµƒјл„”їѓЇѕќп£Ѓ
ґћ÷№∆Џ÷ч„е‘™ЋЎA°ҐB°ҐC°ҐD°ҐEµƒ‘≠„”–т э“јіќ‘ціу£ђЋь√«‘≠„”ЇЋЌвµƒµз„”≤г э÷ЃЇЌќ™10£їB‘™ЋЎµƒїѓЇѕќп÷÷јаЈ±ґа£ђ эƒњ≈”іу£ЃC°ҐDЅљ÷÷‘™ЋЎ–ќ≥…µƒµ•÷ «њ’∆ш÷–ЇђЅњ„оґаµƒќп÷ £їD°ҐEЅљ‘™ЋЎњ…“‘…ъ≥…Ѕљ÷÷≤їЌђµƒјл„”їѓЇѕќп£Ѓ NH3?H2O+H+
NH3?H2O+H+ NH3?H2O+H+
NH3?H2O+H+ 2NO2
2NO2 2NO2
2NO2| 1 |
| 3 |
| 1 |
| 3 |
≤йњіір∞ЄЇЌљвќц>>
њ∆ƒњ£ЇЄя÷–їѓ—І јі‘і£Ї ћв–Ќ£Ї
≤йњіір∞ЄЇЌљвќц>>
њ∆ƒњ£ЇЄя÷–їѓ—І јі‘і£Ї≤їѕк ћв–Ќ£Їќ ірћв
≤йњіір∞ЄЇЌљвќц>>
њ∆ƒњ£ЇЄя÷–їѓ—І јі‘і£Ї2011ƒкƒюѕƒќв÷“ –«аЌ≠ѕњ –ЄяњЉїѓ—Іƒ£ƒв ‘Њн£®»э£©£®љвќц∞ж£© ћв–Ќ£Їљвірћв

≤йњіір∞ЄЇЌљвќц>>
єъЉ —І–£”≈—° - ЅЈѕ∞≤бЅ–±н - ‘ћвЅ–±н
Їю±± °ї•Ѕ™Ќшќ•Ј®ЇЌ≤їЅЉ–≈ѕҐЊў±®∆љћ® | Ќш…ѕ”–Ї¶–≈ѕҐЊў±®„®«ш | µз–≈’©∆≠Њў±®„®«ш | …жјъ Ј–йќё÷ч“е”–Ї¶–≈ѕҐЊў±®„®«ш | …ж∆у«÷»®Њў±®„®«ш
ќ•Ј®ЇЌ≤їЅЉ–≈ѕҐЊў±®µзї∞£Ї027-86699610 Њў±®” ѕд£Ї58377363@163.com