
| A�� | ����������Ϊ40%���ܶ�Ϊ1.43g•cm-3����NaOH��Һ������ˮ��ϣ�������Һ�����ʵ�����������20% | |
| B�� | Ҫ����Ũ��Ϊ0.25mol•L-1��NaOH��Һ480mL��Ӧ����4.8gNaOH��250mL���ձ����ܽ⣬��ȴ����ת�Ƶ�500mL����ƿ�У�ϴ�ӡ�ת�ơ����� | |
| C�� | ����һ�����ʵ���Ũ�ȵ���Һ������ʱ���ӿ̶��ߵ�������Ũ��ƫ�� | |
| D�� | ����NaOH��Һ�����ձ����ܽ�NaOH��δ��ȴ�����¾�ת�Ƶ�����ƿ�У���ҺŨ��ƫ�� |
���� A��ˮ���ܶ�С������������Һ�������ˮ������С������������Һ��������Һ���������Ƶ�������������20%��
B������480mL��Һ��ʵ�������Ƶ���500mL��Һ��
C����ʴ����ƿ�̶��ߣ���������������ƫС��
D���ȵ���Һ���ƫ����ȴ����Һ�����С�����Ƶ���Һ���ƫС��
��� �⣺A������������Ϊ40%���ܶ�Ϊ1.43g•cm-3����NaOH��Һ������ˮ��ϣ�����ˮ���ܶ�С������������Һ��������ˮ������С������������Һ����������Һ�����ʵ�������������20%����A����
B��Ҫ����Ũ��Ϊ0.25mol•L-1��NaOH��Һ480mL��ʵ�������Ƶ���500mL 0.25mol/L��NaOH��Һ��С���������Ƶ�����Ϊ��0.25mol/L��0.5L��40g/mol=5.0g����B����
C������һ�����ʵ���Ũ�ȵ���Һ������ʱ���ӿ̶��ߣ����¼�������������ƫС�����Ƶ���Һ���ƫС��������Ũ��ƫ�ߣ���C��ȷ��
D������NaOH��Һ�����ձ����ܽ�NaOH��δ��ȴ�����¾�ת�Ƶ�����ƿ�У����Ƶ���Һ���ƫС����ҺŨ��ƫ�ߣ���D����
��ѡC��
���� ���⿼��������һ�����ʵ���Ũ�ȵ���Һ�ķ������������е��Ѷȵ����⣬���������ǿ�������߿��������������У�ע������ԣ����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������������ѧ������˼ά�������Ͻ��Ĺ淶ʵ�����������������ѵ�������������ע����ȷ�������ķ�����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ij����ͨ����ˮ�У���ˮ��ɫ��ȥ��������һ������ϩ | |
| B�� | ��������ʱ�¶ȼ�ˮ�����ڷ�ӦҺ�� | |
| C�� | �����Ը��������Һ�ȿɼ���CH4��C2H4���ֿɳ�ȥCH4�е�C2H4 | |
| D�� | ���ᴿ�����еĵ�����ʱ����������Һ�м���Ũ��NH4��2SO4��Һ��Ȼ�����ó����˳������ýϴ��ĵ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1 mol H2��ȫȼ��������̬ˮ���ų�241.8 kJ������H2��ȼ���ȡ�H=-241.8 kJ/mo1 | |
| B�� | ��ϡ��Һ�У�H+��aq��+OH-��aq��=H2O��1����H=-57.3kJ/mo1��������0.5 mol H2SO4��Ũ��Һ�뺬1 mol NaOH����Һ��ϣ��ų�����������57.3kJ | |
| C�� | �Ȼ�ѧ����ʽ�У���ѧʽǰ��Ļ�ѧ�������ɱ�ʾ���������ɱ�ʾ���ʵ��� | |
| D�� | ��֪��C�����ʯ��s��=C��ʯī��s����H��0����˽��ʯ��ʯī�ȶ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���뱽�Ӷ�������ˮ����ȡ����Ӧ | |
| B�� | �����뱽�״���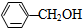 ��������һ��-CH2-ԭ���ţ�������ǻ�Ϊͬϵ�� ��������һ��-CH2-ԭ���ţ�������ǻ�Ϊͬϵ�� | |
| C�� | �ڱ�����Һ�У����뼸��FeCl3��Һ����Һ��������ɫ | |
| D�� | ���ӷ����������ǻ��Ա�����Ӱ�죬ʹ������5����ԭ�Ӷ�����ȡ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 22.4L Cl2��ȫ��Һ����������Һʱ��ת�Ƶ�����ΪNA | |
| B�� | �����£�18g D2O���������ӵ���ĿΪ10NA | |
| C�� | ��״���£�2.24L NH3�к��еĹ��ۼ���ĿΪ0.3NA | |
| D�� | �����£�23g NO2��N2O4�Ļ�������к���NA����ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH2Cl-CH2-CH2Cl | B�� | CHCl2-CH2-CH3 | C�� | CH2Cl-CHCl-CH3 | D�� | CH3-CCl2-CH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1molFeI2��1molCl2��Ӧʱת�Ƶĵ�����Ϊ3NA | |
| B�� | pH=1��H2SO4��Һ����H+����ĿΪNA | |
| C�� | 273K��101kPa�£�28g��ϩ���ϩ������к�����ԭ��������ĿΪ2NA | |
| D�� | 2g H218O��D216O�Ļ�������������ӡ�������Ŀ��ΪNA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ʵ�� | �¶�/�� | ��ʼʱ | ƽ��ʱ | |||
| n��CO��/mol | n��H2S��/mol | n��COS��/mol | n��H2��/mol | n��CO��/mol | ||
| 1 | 150 | 10.0 | 10.0 | 0 | 0 | 7.0 |
| 2 | 150 | 7.0 | 8.0 | 2.0 | 4.5 | a |
| 3 | 400 | 20.0 | 20.0 | 0 | 0 | 16.0 |
| A�� | ������Ӧ�����ȷ�Ӧ | |
| B�� | ʵ��1��ƽ��ʱ��CO��ת����Ϊ70% | |
| C�� | ʵ��2��ƽ��ʱ��a��7.0 | |
| D�� | ʵ��3��ƽ����ٳ���1.0 mol H2��Kֵ����ƽ�������ƶ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com