
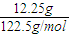 ×��5-0��=0.5mol���ʴ�Ϊ��0.5��
×��5-0��=0.5mol���ʴ�Ϊ��0.5��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ����� | �Լ� | ���뷽�� | |
| �� | ����ؾ��壨�Ȼ��ƣ� | ˮ | |
| �� | ���飨��ϩ�� | ϴ�� | |
| �� | �������Ҵ��� | ||
| �� | ���Ȼ�̼��ˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ��ȥ�����������������������ʣ�������Ϊ���ʣ�д�������Լ��ͷ�Ӧ�����ӷ���ʽ��
��1��FeSO4��Fe2(SO4)3���Լ�_________��
���ӷ���ʽ____________________________________��
��2��
FeCl3(FeCl2)�Լ�_________�����ӷ���ʽ_______________________________________��
��3��
NaCl��Һ��FeCl3���Լ�_________�����ӷ���ʽ___________________________________��
��4��
Fe2O3(SiO2)�Լ�_________�����ӷ���ʽ_________________________________________��
��5��
Fe�ۣ�Al�ۣ��Լ�_________�����ӷ���ʽ____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(��14��)
I . Ϊ��ȥ���������������е��������ʣ�д��ѡ����Լ���д���й����ӷ���ʽ��
��1�������л�������SiO2����ѡ���Լ� ��
���ӷ���ʽ ��
��2��FeCl3��Һ�л�������FeCl2����ѡ���Լ� ��
���ӷ���ʽ ��
II . ������H2SO4���ʵ���ţ������С������Ӧ�ĺ����ϡ�
A����ˮ�� B��ǿ���� C���ѻӷ��� D�������� E����ˮ��
��1����ŨH2SO4���Ը���SO2����
��2�����ձ��з������ǣ�����ŨH2SO4�����ɫ
��3�������ŨH2SO4�з�����Ƭû����������
III . KClO3��Ũ�����ڳ����·�Ӧ����������KClO3+6HCl��Ũ��= KCl+ 3Cl2��+ 3H2O
��1���������� ����ԭ������ ��
��2������12.25g KClO3������Ӧ����ת�Ƶĵ��ӵ����ʵ���Ϊ mol
��3������������ʹ�������ɫʯ����ֽ�ȱ������ɫ���� ������ţ�
������ ��Һ�� ��������ˮ �ܳ��ڷ��õľ�����ˮ������ ���˴����Ư����Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014������и�һ��ѧ����ĩ������ѧ�Ծ� ���ͣ������
(��14��)
I . Ϊ��ȥ���������������е��������ʣ�д��ѡ����Լ���д���й����ӷ���ʽ��
��1�������л�������SiO2����ѡ���Լ� ��
���ӷ���ʽ ��
��2��FeCl3��Һ�л�������FeCl2����ѡ���Լ� ��
���ӷ���ʽ ��
II . ������H2SO4���ʵ���ţ������С������Ӧ�ĺ����ϡ�
A����ˮ�� B��ǿ���� C���ѻӷ��� D�������� E����ˮ��
��1����ŨH2SO4���Ը���SO2����
��2�����ձ��з������ǣ�����ŨH2SO4�����ɫ
��3�������ŨH2SO4�з�����Ƭû����������
III . KClO3��Ũ�����ڳ����·�Ӧ����������KClO3+6HCl��Ũ��= KCl+ 3Cl2��+ 3H2O
��1���������� ����ԭ������ ��
��2������12.25g KClO3������Ӧ����ת�Ƶĵ��ӵ����ʵ���Ϊ mol
��3������������ʹ�������ɫʯ����ֽ�ȱ������ɫ���� ������ţ�
������ ��Һ�� ��������ˮ �ܳ��ڷ��õľ�����ˮ ������ ���˴����Ư����Һ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com