
2009��12��18�շ��������������������������ҽר�ҳ���7���µĿ��й��أ������Ƴ�һ����Ч���Ƽ���H1N1���е�����ҩ������з���������Ҫ�ɷ�֮һ�ǽ�������������������Ч�ɷ�֮һ����ԭ�ᡣ�������й���ԭ��ķ��ӽṹ����ת����ϵ������EΪ���������䱽���ϵ�һ�ȴ���ֻ��һ�֣����ֲ���ʡ�ԣ�


��ش��������⣺
��1����ԭ��ķ���ʽ�� ��A�к��������ŵ�����Ϊ ��H�Ľṹ��ʽΪ ��
��2���ٵķ�Ӧ������ ��1mol��ԭ��������� molNaOH��
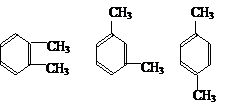
��3��������E��ͬ���칹�壨��E������ͬ��ȡ�������ķе��ɴ�С��˳���ǣ�����E���ڣ� ���ýṹ��ʽ��ʾ����
��4��д����Ӧ�ۡ��ܵĻ�ѧ����ʽ��
�� ��
�� ��
��5��B�ǿ����ᣬ���ж���ͬ���칹�壬д��������������������ͬ���칹��Ľṹ��ʽ����������a.�����Ϻ�������ȡ�������ұ����ϵ�һ�ȴ���ֻ�����֣�b.�ܷ���ˮ�ⷴӦ���Ҳ���֮һ�ܷ���������Ӧ��c.����̼��������Һ��Ӧ�� ��
��1��C16H18O9(1��)�Ȼ����������ǻ���1�֣�
 |
��2�֣�
��2��ˮ�ⷴӦ����ȡ����Ӧ����1�֣�4��2�֣�
 ��3��
��3��
�� �� ��2�֣�
 |
![]() ��4��
��4��
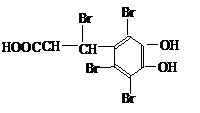
![]() +4Br2 +3HBr
+4Br2 +3HBr
(2��)
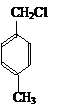

![]() + NaOH +NaCl (2��)
+ NaOH +NaCl (2��)
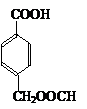 |  |
��5��
���������µ�ҽҩ��չΪ�������漰±�����������ӡ��ᡢ���������Լ���ѧ����ʽ����Ӧ���͡�ͬ���칹�����д����Ҫ֪ʶ���ۺϿ��顣��1����֪��ԭ����ӵĽṹʽ������̼ԭ�Ӻ���ԭ�Ӽ���д������ʽ����ԭ����ϡ����������·���ˮ�ⷴӦ�����ɵ�AΪ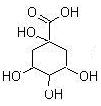 ��BΪ ����ΪA��B��B����Ũ��ˮ����
��BΪ ����ΪA��B��B����Ũ��ˮ����
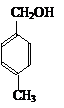 |
 ��Ӧ������֪EΪ�Զ��ױ���FΪ ��GΪ �����Ƴ�H�Ľṹ��ʽΪ
��Ӧ������֪EΪ�Զ��ױ���FΪ ��GΪ �����Ƴ�H�Ľṹ��ʽΪ
���ڣ�3���ʿ���ѧ���Խ̲ĵ���Ϥ�̶ȣ�
�е㣺�ڶ��ױ�������ױ����Զ��ױ����ڣ�4������д��ѧ����ʽҪע�ⷴӦ��������ƽ���ž��淶��д��ʧ�ֵ�������ڣ�5������дͬ���칹��ʱ��Ҫע��������������Ҫ©д��Ҳ��Ҫ��д��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
| ||
| ̫���� |
| ���� |
| 7 |
| 448 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
| ||
| ���� |
| ���� |
| C(HCOOH)C(OH-) |
| C(HCOO-) |
| C(HCOOH)C(OH-) |
| C(HCOO-) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �籾��������������ȫ�ơ����Ϲ�����仯��ܹ�Լ����15�ε�Լ�������ߡ������鶨�顷��5�ε�Լ�����飬��2009��12��7��18���ڵ������籾�����ٿ�������192�����ҵ�̸�д����ٿ���ᣬ���֡������鶨�顷һ�ڳ�ŵ���ں�ĺ�����������2012��2020���ȫ�����Э�飮
�籾��������������ȫ�ơ����Ϲ�����仯��ܹ�Լ����15�ε�Լ�������ߡ������鶨�顷��5�ε�Լ�����飬��2009��12��7��18���ڵ������籾�����ٿ�������192�����ҵ�̸�д����ٿ���ᣬ���֡������鶨�顷һ�ڳ�ŵ���ں�ĺ�����������2012��2020���ȫ�����Э�飮| ��� | Ԥ������ | ʵ����� |
| A | ||
| B | ||
| C |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com